Summary
Estradiol regulates serotonin 1A(5-HT1A) receptor signaling. Since desensitization of 5-HT1A receptors may be an underlying mechanism by which selective serotonin reuptake inhibitors (SSRIs) mediate their therapeutic effects and combining estradiol with SSRIs enhances the efficacy of the SSRIs, it is important to determine which estrogen receptors are capable of desensitizating 5-HT1A receptor function. We previously demonstrated that selective activation of the estrogen receptor, GPR30, desensitizes 5-HT1A receptor signaling in rat hypothalamic paraventricular nucleus(PVN). However, since estrogen receptor beta(ERβ), is highly expressed in the PVN, we investigated the role of ERβ in estradiol-induced desensitization of 5-HT1A receptor signaling. We first showed that a selective ERβ agonist, diarylpropionitrile(DPN) has a 100-fold lower binding affinity than estradiol for GPR30. Administration of DPN did not desensitize 5-HT1A receptor signaling in rat PVN as demonstrated by agonist-stimulated hormone release. Second, we used a recombinant adenovirus containing ERβ siRNAs to decrease ERβ expression in the PVN. Reductions in ERβ did not alter the estradiol-induced desensitization of 5-HT1A receptor signaling in oxytocin cells. In contrast, in animals with reduced ERβ, estradiol administration, instead of producing desensitization, augmented the ACTH response to a 5-HT1A agonist. Combined with the results from the DPN treatment experiments, desensitization of 5-HT1A receptor signaling does not appear to be mediated by ERβ in oxytocin cells, but that ERβ, together with GPR30, may play a complex role in central regulation of 5-HT1A-mediated ACTH release. Determining the mechanisms by which estrogens induce desensitization may aid in the development of better treatments for mood disorders.
Keywords: estrogen receptors, neuroendocrine responses, siRNA, recombinant adenovirus, serotonin receptors, mood disorders, estradiol
Introduction
Women past the age of 40 begin to experience fluctuation, then a decline in the levels of estrogens resulting in a peri- to post-menopausal state (Banger, 2002). Decreased levels of estrogens are associated with various neuropsychiatric disorders such as depression, anxiety, and panic disorders in women (Arpels, 1996). One particular hallmark of these disorders is a change in serotonergic function (Halbreich, 1990; Jimerson et al., 1997; Joffe and Cohen, 1998; Menkes et al., 1994; Ressler and Nemeroff, 2000), and particularly in serotonin 1A (5-HT1A) receptor function (Lemonde et al., 2003; Savitz et al., 2009; Shively and Bethea, 2004).
Several groups have shown that estrogen treatment modulates 5-HT1A receptor signaling. There is evidence for both species and regional differences in the regulation of 5-HT1A receptors following estrogen treatment. In rat studies, either a single injection or two-three days of estrogen treatment results in a decrease in 5-HT1A receptor gene expression (Osterlund and Hurd, 1998), G protein coupling (Mize and Alper, 2000), and 5-HT1A receptor function (D'Souza et al., 2004; Jackson and Uphouse, 1996, 1998; Raap et al., 2000) in the limbic region, cortex, and dorsal raphe nucleus. Similarly, chronic estrogen treatment in nonhuman primates and rats resulted in a decrease in 5-HT1A receptor gene expression (Birzniece et al., 2001), 5-HT1A receptor density, and 5-HT1A receptor-G protein coupling in the dorsal raphe nucleus (Lu and Bethea, 2002; Pecins-Thompson and Bethea, 1999). Following chronic estrogen treatment, 5-HT1A receptor gene expression (Birzniece et al., 2001) and 5-HT1A receptor-G protein coupling in cortical and hippocampal regions decreased without changes in 5-HT1A receptor density in rats (Le Saux and Di Paolo, 2005). In contrast, in nonhuman primates there was no evidence for changes in 5-HT1A receptor gene expression (Gundlah et al., 1999) or 5-HT1A receptor-G protein coupling in the hypothalamus (Lu and Bethea, 2002). The regional differences may be indicative of different estrogen receptor subtypes involved in the regulation of 5-HT1A receptors.
Understanding which estrogen receptor is involved in the regulation of hypothalamic 5-HT1A receptors may be important for improving the current therapies for mood disorders. Clinical studies have demonstrated that combining estrogen with SSRIs enhances the efficacy of the SSRIs in treating women with mood disorders and hot flushes (Schneider et al., 1997), but the mechanism by which this occurs is not known. Desensitization of somatodendritic 5-HT1A autoreceptors in the midbrain and postsynaptic 5-HT1A receptors in the hypothalamus are considered to be a likely underlying mechanism by which antidepressants, particularly SSRIs, mediate their therapeutic effects (Bosker et al., 2001; Chaput et al., 1986; Czachura and Rasmussen, 2000; Kreiss and Lucki, 1995). In contrast to SSRI administration which takes one-two weeks to produce a full desensitization (Li et al., 1997; Li et al., 1996), beta-estradiol 3-benzoate (EB) treatment results in partial desensitization of the 5-HT1A receptor signaling in the paraventricular nucleus of the hypothalamus (PVN) within 2 days (D'Souza et al., 2004; Raap et al., 2000).
Activation of the post-synaptic 5-HT1A receptors in the PVN stimulates the release of the hormones oxytocin, adrenocorticotropin hormone (ACTH), and corticosterone (Osei-Owusu et al., 2005). Based on our previous study of co-localization of estrogen receptors with 5-HT1A receptors, oxytocin and CRH, we know that 5-HT1A receptors co-localize with both oxytocin and CRH in the PVN (Xu et al., 2008). Oxytocin is a neurohormone with anxiolytic and antidepressant activity and it is important in humans for socialization (Gimpl and Fahrenholz, 2001) whereas ACTH and corticosterone are pituitary and adrenal hormones respectively, associated with stress (Carrasco and Van de Kar, 2003). Measuring these hormones following activation of 5-HT1A receptors provides an indication of desensitization of 5-HT1A receptor activity in the PVN and can be used to assess the delay in the desensitization response to EB and other drug treatments.
We recently found that the G protein coupled estrogen receptor, GPR30, is involved in the desensitization of the 5-HT1A receptor signaling in the PVN (Xu et al., 2009). In addition to GPR30, PVN neurons also express the nuclear estrogen receptors, estrogen receptor alpha (ERα) and beta (ERβ). The density of ERβ is much higher than that of ERα in the PVN (Laflamme et al., 1998), especially in oxytocin neurons (Alves et al., 1998; Hrabovszky et al., 2004; Simonian and Herbison, 1997). Therefore in the current study, we focused on whether ERβ is involved in the EB-induced desensitization of the 5-HT1A receptors in the PVN. We used two complimentary approaches to address this question. In one experiment, we asked whether a selective ERβ agonist, diarylpropionitrile (DPN) desensitizes 5-HT1A receptors in the PVN. In a second complimentary experiment, we decreased the expression of the ERβ by injecting a recombinant adenovirus containing a small interference RNA (siRNA) against the ERβ into the PVN and then evaluated the effects of EB on 5-HT1A receptor signaling.
Methods
Animals
Female Sprague-Dawley rats (225–275g) from Harlan (Indianapolis, IN) were housed two per cage in a temperature-, humidity-, and light-controlled room (12h light/dark cycle, lights on 0700 h). Food and water were available ad libitum. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication 85-23, revised 1996) and as approved by the University of Kansas Institutional Animal Care and Use Committee. All efforts were made to minimize animal suffering and to reduce the number of animals used.
Drugs
EB was purchased from Sigma Research Biomedical Inc. (St. Louis, MO). DPN and (+)-8-hydroxy-2-dipropylaminotetralin hydrobromide ((+)8-OH-DPAT) were purchased from Tocris (Ellisville, MO). (+)8-OH-DPAT was dissolved in saline at a concentration of 0.2 mg/ml and administered at a dose of 0.2 mg/kg, sc. At this near maximal effective dose of (+)8-OH-DPAT, the 5-HT1A receptor antagonist WAY-100635 blocks the hormone response by (+)8-OH-DPAT suggesting that the effect is due to selective activation of 5-HT1A receptors over 5-HT7 receptors at this dose (Critchley et al., 1994; Vicentic et al., 1998). EB was first dissolved in 100% ethanol to a concentration of 1 mg/ml and then diluted with sesame oil to a concentration of 5 µg/ml. The EB solution and sesame oil were administrated at 0.4 ml/kg (EB dose 2 µg/kg, sc). DPN was first dissolved in 100% DMSO to a concentration of 40 mg/ml, and then this solution was diluted to 2 mg/ml with 25% DMSO/saline (administrated at 1 ml/kg, sc). The DPN dose was chosen based on previous studies (Le Saux et al., 2006; Lund et al., 2005; Mazzucco et al., 2006). All of these solutions were made fresh before injection.
Experiment 1: Effect of the selective estrogen receptor beta (ERβ) agonist, DPN, on 5-HT1A receptor function
DPN Binding to GPR30
DPN is a selective ERβ agonist with an approximately 100-fold higher affinity for ERβ than estrogen receptor alpha (ERα) (Lund et al., 2005). However, no data were available regarding the affinity of DPN for GPR30. Since GPR30 can mediate EB-induced desensitization of 5-HT1A receptors (Xu et al., 2009), it was necessary to determine the relative affinity of DPN for GPR30. DPN binding to human recombinant GPR30 was examined by a membrane estrogen radioreceptor assay described previously (Thomas et al., 2005). Plasma membranes of human recombinant GPR30-transfected HEK293 cells were prepared by homogenization with a glass homogenizer followed by sequential centrifugation steps of 1000×g for 7 minutes and 20,000×g for 20 min (Pang et al., 2008). An aliquot (0.125mg protein in 250µl) of the resulting plasma membrane pellet was incubated for 30 minutes with 2 nM of [3H]-estradiol-17β ([3H]-E2) in the presence of E2 or DPN at the concentrations 10−10- 10−5 M. The bound [3H]E2 was separated from free [3H]E2 using glass-fiber filters. Competition binding was expressed as a percentage of maximum specific binding. The competitive binding assay was repeated three times.
Effect of DPN treatment on 5-HT1A receptor function
Rats were ovariectomized by removing both ovaries via a single ventral midline incision. Prior to surgery, an intraperitoneal injection of a mixture of ketamine hydrochloride (100 mg/kg) plus xylazine hydrochloride (10 mg/kg) was used to anesthetize the animals. Five days after surgery, animals were given subcutaneous (sc) injections of either DPN (2 mg/kg) or 25% DMSO/saline (vehicle) once a day for two days. A 5-HT1A receptor agonist, (+)8-OH-DPAT (0.2 mg/kg, sc) was injected 18 hours following the last injection of DPN or vehicle. Fifteen minutes later, animals were sacrificed by decapitation. Trunk blood was collected in centrifuge tubes containing 0.5 ml EDTA (0.3 M, pH 7.4). Plasma was stored at −80°C until radioimmunoassays were conducted.
Experiment 2: Effect of recombinant adenovirus containing ERβ siRNA on EB-induced desensitization of 5-HT1A receptors
Generation of recombinant adenoviruses containing ERβ siRNAs
To identify the potential siRNAs for ERβ, four ERβ siRNAs were selected for initial testing from a list of ERβ siRNAs suggested by a siRNA design program siDesign Center (Dharmacon, Inc., Thermo Fisher Scientific, Lafayette, CO). The sense sequences of the ERβ siRNAs were siRNA1:UCGCAAGUGUUAUGAAGUAUU; siRNA2:GCACAAGGAGUAUCUCUGUUU; siRNA3:AAUCAUCGCUCCUCUAUGCUU and siRNA4:GUCAAAGGUUCCGUGAGUUAUU. ERβ siRNA duplexes (Dharmacon, Inc., Thermo Fisher Scientific, Lafayette, CO) were transfected into PC-12 cells. The levels of ERβ mRNA in the cells were determined 48 hours later using RT-PCR.
Based on the extent of ERβ mRNA reduction, selected ERβ siRNAs were further evaluated using a pSOS-HUS vector (provided by Dr. TC He at the University of Chicago). The pSOS-HUS contains an siRNA site and a target gene site that allows transfection of siRNAs and the target gene into the same cells. The target gene is fused with an enhanced green fluorescent protein (GFP) gene, so that the expression of the target gene can be directly observed by fluorescence microscopy. The rat ERβ coding region was amplified by PCR with primers encoding 417–441 (Forward: ATGACATTCTACAGTCCTGCTGTG) and 1874-1851 (reverse: TCACTGAGACTGTAGGTTCTGGG) of ERβ cDNA sequence (Accession # NM_012754). The PCR product was then inserted into the target site of pSOS-HUS and fused with GFP (SOSERβ-HUS). After digestion with Sfi I at the siRNA insertion site of SOS-ERβ-HUS, a double-stranded DNA oligonucleotide encoding ERβ siRNA1 or 2 sequences was ligated into SOS-ERβ-HUS to generate SOS-ERβ-siRNA-HUS.
The SOS-ERβ-siRNA-HUSs or SOS-ERβ-HUS was then transfected into HEK 293 cells to evaluate the knockdown efficiency of the ER-beta siRNAs. When comparing the SOS-ERβ-siRNA-HUSs transfected cells with those transfected with SOS-ERβ-HUS, a reduction in the number and density of GFP-containing cells indicates the efficiency of knockdown by the siRNA. In a separate experiment, the SOS-ERβ-siRNA-HUSs or SOS-ERβ-HUS was transfected into PC12 cells. 48 hours later, the cells were collected and lysed. The ERβ levels in the cell lysis were examined by western blot.
The siRNAs in the SOS-ERβ-siRNA-HUS that reduced the percentage and brightness of GFP-containing cells were selected and inserted into pSES-HUS vector (a shuttle vector for adenovirus). The SES-ERβ-siRNA-HUSs were further recombined into Ad-Easy-1 vector to generate high titer adenoviruses containing the siRNAs as described by Luo et al (He et al., 1998; Luo et al., 2007) and our previous publication (Li et al., 2004). Red fluorescent protein (RFP) is expressed independently along with the siRNAs by the adenoviral vector. RFP can be used as a marker for viral infection. The high titer ERβ-siRNA1-Ad and ERβ-siRNA2-Ad (~1010 infectious viral particles/ml) were stored at −80°C. To prevent tissue damage induced by the high salt storage-solution, the high titer recombinant adenovirus ERβ-siRNA-Ads (about 30 µl) were dialyzed with saline (about 1L) for at least 40 min at 4°C followed by 1:1 dilution with saline before use.
To evaluate ERβ-siRNA-Ads in vivo, the high titer ERβ-siRNA-Ads were unilaterally injected into the PVN using stereotaxic technique at a rate of 0.2 µl/min (1 µl/side at the coordinates of AP= −1.8, ML= 0.5 and DV= −8.3 mm with respect to bregma (Paxinos and Watson, 2007). The needle was left at the site of injection for 20 minutes to reduce movement of the viral solution into the needle track. 7 and 10 days after the injection, rats were decapitated and the brains were collected to evaluate the efficiency of knockdown of ERβ expression by the siRNAs. Regions with viral infection as indicated by RFP and the contra-lateral regions (as controls) were punched for immunoblot analysis of ERβ.
Effect of ERβ-siRNA-Ads on EB-induced desensitization of 5-HT1A receptors
After demonstrating that ERβ expression was decreased by the virus containing siRNAs for ERβ, the next experiment was to test the effect of ERβ knockdown on 5-HT1A receptor function. In this experiment, rats were anesthetized and ovariectomized as described above in Experiment 1. On the same day as the ovariectomy, either a control adenovirus (Ad-track, 1µL/side) or ERβ siRNA-Ads (mixed ERβ-siRNA1-Ad and ERβ-siRNA2-Ad, 1µL/site) was bilaterally microinjected into the PVN as described above. The control adenovirus (Ad-track) was generated by recombination of an empty Ad-track shuttle vector with the adenoviral vector.
On days 5 and day 6 after ovariectomy and viral injections, rats were injected with EB (2µg/kg, 0.4ml/kg) or sesame oil subcutaneously once a day for 2 days. To assess 5-HT1A receptor signaling in the PVN, the 5-HT1A receptor agonist, (+)8-OH-DPAT (0.2 mg/kg, sc) was injected 18 hours after the last EB or oil injection. Animals were sacrificed 15 minutes after the (+)8-OH-DPAT injection as described in Experiment 1. Trunk blood was collected for hormone radioimmunoassays. The brains were removed and flash-frozen in isopentane chilled over dry ice, and stored at −80°C until further analysis.
Coronal brain sections were cut in a cryostat microtome to obtain a 700 µm thick section at the level of the PVN (Paxinos and Watson, 2007). The PVN was dissected for western blot analysis of ERβ receptors. In addition, 16 µm thick sections, before and after the 700 µm PVN section, were collected and mounted on microscope slides. These sections were used to determine whether the adenovirus was correctly microinjected into the PVN.
Immunoblot assay for ERβ receptors
The dissected brain tissues were homogenized in buffer (10 mM Tris, pH7.4, 100 mM NaCl, 1mM EDTA, 1 mM DTT, 0.1% sodium cholate and 1× protease inhibitor cocktail) by sonication 5 sec × 3 time with 30 sec intervals in ice. After centrifugation at 25,000 × g, 4°C, the supernatant was used for western blots. The protein concentration was determined using a bicinchoninic acid protein assay kit (Thermo Fisher Scientific, Rockford, IL).
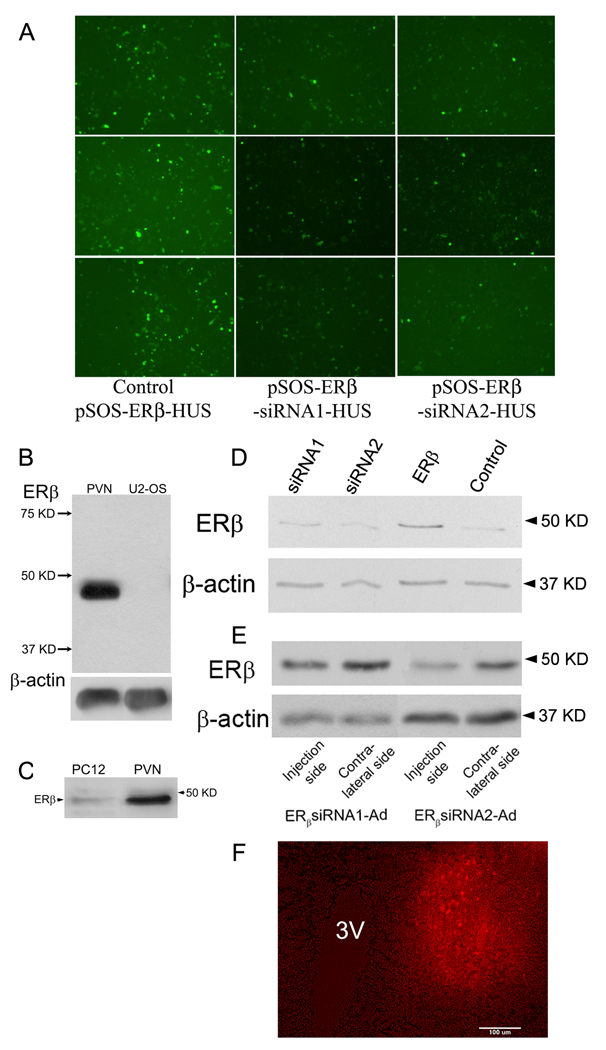
ERβ levels in cell lysates or brain homogenates were measured using immunoblot analysis. The proteins (3 µg/lane) were resolved on a 12% SDS-page gel followed by transferred to a PVDF membrane. The membranes were then incubated with blocking buffer (0.2% I-block (Tropix, Bedford, MA), 0.1% Tween 20 in Tris-buffered saline) followed by rabbit-anti ERβ (1:1000, Alexis Biochemicals, San Diego, CA) overnight at 4°C. The specificity of the ERβ antibody was demonstrated in our experiments in which a single band (~48 KD) was detected on western blots. The intensity of the 48 KD band was increased with over-expression in PC12 cells (Figure 3D), and no bands were detected in U-2 OS cell lysates (Figure 3B), a cell line that does not express ERβ (Levy et al., 2009). Moreover, the size of the band detected in PC12 cells was the same as in the PVN (Figure 3C). After washes, the membranes were incubated with a HRP-conjugated goat anti-rabbit IgG (Jackson Immunoresearch, West Grove, PA, 1:10,000). The bands were detected with ECL substrate solution (GE Healthcare Biosciences Corp, Piscataway, NJ) on x-ray film. The membranes were washed and then incubated with monoclonal mouse actin antibody (MP Biomedicals, Solon, OH, 1:10,000) and processed for the detection of actin immunoreactivity.
Figure 3.
Selection and evaluation of ERβ siRNAs. A. Selection of ERβ siRNA1 and 2 in SOS-HUS vector. SOS-ERβ-siRNA-HUSs or SOS-ERβ-HUSs were transfected into HEK 293 cells. The number and brightness of GFP-containing cells were compared between SOS-ERβ-siRNA-HUSs and SOS-ERβ-HUS transfected cells. Both vectors express GFP-tagged ERβ so a decrease in GFP intensity in the cells transfected with SOS-ERβ-siRNA-HUSs indicates a decrease in ERβ expression due to the siRNA. Each construct was transfected into three wells which are represented in each column. B. Identifying the selectivity of ERβ antibody: an immunoblot prepared with the ERβ antibody and homogenate from rat PVN (5 µg protein) and a negative control, lysates from U-2 OS cells (5 µg protein) which do not express ERβ. The blot was then reprobed with an actin antibody to demonstrate equal loading of protein in each lane. C. Immunoblot prepared with the ERβ antibody demonstrates that a single band at approximately 48 KD was detected in both the homogenate from rat PVN and PC12 cells lysates. D. Immunoblot for ERβ from PC12 cells transfected with different constructs. siRNA1, siRNA2 and ERβ represent the cells transfected with SOS-ERβ-siRNA1-HUS, SOS-ERβ-siRNA2-HUS and SOS-ERβ-HUS, respectively. The control designation indicates non-transfected cells. E. Example of an immunoblot for evaluation of the knockdown effect of ERβ with virally expressed siRNA in vivo. High titer ERβ-siRNA1-Ad and ERβ-siRNA2-Ad were unilaterally injected into the PVN of rats. ERβ levels in the injected side were compared with the contralateral side. F. An example of RFP labeling in the PVN to show the position of the viral injection. The images were captured from a fresh frozen brain section. The left panel was captured under fluorescent light, while the right panel was captured under bright field and fluorescent light combined in order to show the location of injection relative to the third ventricle (3V). Calibration bar indicates 100 microns.
Films from the western blots were analyzed densitometrically using MCID Basic Program version 7.0 (Interfocus Imaging Ltd., Cambridge, England). The integrated optical density of protein bands were calculated as previously described (Raap et al., 2000). The density of the ERβ bands was normalized to the density of the β-actin band in the same sample. The percent reduction of ERβ expression induced by ERβ-siRNA-Ads during preliminary testing was calculated by comparing ERβ expression on the side injected with virus to the contralateral side. To determine the effect of ERβ-siRNA-Ads on EB-induced desensitization of 5-HT1A receptors, ERβ expression was normalized to actin expression in the sample, then divided by the mean of the control animals for each immunoblot and expressed as a percent mean of the control samples.
Radioimmunoassay of plasma oxytocin and ACTH
Plasma oxytocin was determined by a radioimmunoassay as previously described with minor modifications (Li et al., 1997). Briefly, oxytocin was extracted from 0.5 mL plasma with 1 mL cold acetone followed by 2.5 mL of petroleum ether (Li et al., 1997). The ether layer (top layer) was aspirated and the samples were dried in a Centrivap vacuum concentrator at 4°C. The dried oxytocin residue was resuspended in 1mL of cold assay buffer (0.05M phosphate buffer pH7.4 containing 0.125% bovine serum albumin and 0.001M EDTA). The plasma extract (100 or 300 µL) was used for the radioimmunoassay as previously described. The radioactive 125I oxytocin (specific activity: 2200 Ci/mmol) was obtained from Perkin Elmer (Waltham, MA). Several standard recovery samples containing 0.5 ml pooled plasma and 8 pg or 16 pg oxytocin were included through the extraction and assay procedure. The plasma oxytocin was calculated based on the recovery and dilution factors. Plasma ACTH concentrations were determined by radioimmunoassay as previously described (Li et al., 1993).
Statistical analysis
All data are expressed as means ± SEM. Oxytocin and ACTH data were analyzed by a two-way analysis of variance (ANOVA) for the DPN experiments or three-way ANOVA for the estrogen siRNA viral injection experiments and a Student-Newman Keuls post hoc test (Statview version 5.0 software, SAS Institute Inc., Cary, NC). Animals with misplaced injections or that were injected with the siRNA construct for ERβ but did not have a decrease in ERβ expression were excluded from the data analysis.
Results
Experiment 1: Effect of selective estrogen receptor beta (ERβ) agonist, DPN, on 5-HT1A receptor function
DPN has a low affinity for GPR30
To determine the affinity of DPN for GPR30, we conducted a competitive 3H-E2 binding assay using cell membranes prepared from HEK293 cells transfected with human GPR30. As shown in figure 1, E2 is able to completely inhibit the 3H-E2 binding to GPR30 with a Ki ~ 10−7M. The competition curve of DPN was shifted to the right relative to E2. The 3H-E2 binding was not completely blocked even with 10−5M of DPN. These results suggest that the affinity of DPN to GPR30 is at least 100 fold lower than E2.
Figure 1.
Example of competition curves of EB and DPN binding in plasma membranes of HEK293 cells stably transfected with GPR30. The data were expressed as a percentage of maximum specific [3H]E2 binding.
Effect of DPN treatment on 5-HT1A receptor function
Two days of treatment with the ERβ agonist, DPN, did not alter the baseline levels of plasma oxytocin in comparison to the vehicle-treated animals (Figure 2A). Activation of 5-HT1A receptors by (+)8-OH-DPAT increased plasma oxytocin levels as expected, but DPN treatment did not alter this increase (Figure 2A). The two-way ANOVA for plasma oxytocin indicated a significant effect of (+)8-OH-DPAT injection (F(1, 35) = 218.7, p<0.0001), but no significant effect of DPN treatment (F(1, 35) = 0.13, p= 0.72) and no significant interaction between DPN treatment and (+)8-OH-DPAT injection (F(1,35) = 0.095, p=0.76).
Figure 2.

Oxytocin and ACTH response to (+)8-OH-DPAT in DPN- or vehicle-treated animals. A. Plasma oxytocin and B. plasma ACTH levels expressed as the mean ± SEM for 7–10 animals. *Significant difference from saline injected animals, p<0.05 by Student-Newman-Keuls post hoc test.
Baseline plasma ACTH levels were not different in animals treated with DPN in comparison to the vehicle-treated animals (Figure 2B). As expected, 5-HT1A receptor activation by (+)8-OH-DPAT increased plasma ACTH levels, but DPN treatment had no effect on the (+)8-OH-DPAT-induced increase in the plasma ACTH (Figure 2B). The two-way ANOVA for plasma ACTH indicated a significant effect of (+)8-OH-DPAT injection (F(1, 35) = 304.7, p<0.0001), but no significant effect of DPN treatment (F(1, 35) = 0.45, p= 0.51) and no significant interaction between DPN treatment and (+)8-OH-DPAT injection (F(1,35) = 0.48, p=0.5).
Experiment 2: Effect of recombinant adenovirus containing ERβ siRNA on EB-induced desensitization of 5-HT1A receptors
Selection and evaluation of ERβ-siRNA-Ads
Of the four ERβ siRNAs tested in PC12 cells, siRNA1 and 2 reduced ERβ mRNA more than 75%, whereas the siRNA3 and 4 only reduced ERβ mRNA about 20% (data not shown). Therefore, we selected the ERβ siRNA1 and 2 for further analysis. SOS-ERβ-siRNA-HUSs and SOS-ERβ-HUS were generated and transfected into HEK 293 cells. As shown in Figure 3A, the number and brightness of GFP-containing cells in SOS-ERβ-siRNA-HUS-transfected cells is significantly lower than that in SOS-ERβ-HUS-transfected cells. Consistent with these results, we also found that ERβ in the SOS-ERβ-siRNA-HUS-transfected cells was reduced relative to the SOS-ERβ-HUS-transfected cells using an immunoblot assay (Fig. 3D). Thus, the ERβ siRNA1 and 2 were selected for production of ERβ siRNA recombinant adenovirus.
To test whether high titer ERβ-siRNA-Ads are able to reduce the ERβ expression in vivo, we unilaterally injected the high titer ERβ-siRNA-Ads into the PVN. As shown in Figure 3E, the ERβ levels in the ERβ-siRNA-Ad injected sides were lower than that in the contralateral uninjected sides. The percent of inhibition was within the range of 47% to 81% (n=5). The reduction of ERβ expression was slightly larger seven days than ten days after viral injection (data not shown).
ERβ in PVN following ERβ-siRNA-Ads injection and EB treatment
Viral injections were monitored by the presence of RFP in the PVN as shown for one animal in Fig. 3F. Animals with incorrect injections were not included in subsequent analyses. We used both the ERβ levels in the PVN and the location of RFP expression as exclusion criteria for “incorrect injections”of ERβ-siRNA-Ads. Rats that did not express RFP in the PVN were excluded first (< 10%). We also excluded the rats for which the reduction of ERβ was less than 20%. The overall rate of successful injection was ~75%. Among rats with incorrect injections, the hormone responses to 8-OH-DPAT were similar to those Ad-track viral injected rats. No changes in the basal hormone levels or other behaviors were observed in the rats with incorrect injections.
Alterations in ERβ after EB treatment and/or ERβ-siRNA-Ads injection were measured using immunoblot analysis (Figure 4). Two days of EB treatment decreased ERβ protein levels in the PVN by 33% in animals microinjected with the control adenovirus. Microinjection with the ERβ-siRNA-Ads resulted in 34% decrease in ERβ protein levels. In animals treated with both ERβ-siRNA-Ads and EB, ERβ protein levels decreased by 50% in the PVN. The two-way ANOVA for ERβ protein levels indicated a significant effect of the viral injection (F(1, 34) = 18.1, p = 0.0002) and significant effect of EB treatment (F(1, 34) = 16.9, p = 0.0002), but no significant interaction between the viral injection and EB treatment (F(1,34) = 1.81, p=0.19).
Figure 4.

Effect of ERβ- siRNA-Ads and EB injection on ERβ protein levels in the PVN. A. An example of an immunoblot of ERβ and β-actin. B. Western blot data analysis of ERβ. Data are expressed as mean ± SEM for 9–10 animals. *Significant difference from control virus/oil injected animals, p<0.05 by Student-Newman-Keuls post hoc test.
Effect of ERβ-siRNA-Ads on EB-induced desensitization of 5-HT1A receptors
There was no difference in the baseline levels of plasma oxytocin in control virus-injected or ERβ-siRNA-Ads-injected animals (Figure 5A). (+)8-OH-DPAT increased plasma oxytocin levels in the control viral-injected and ERβ-siRNA-Ads-injected groups. EB treatment decreased the oxytocin response to (+)8-OH-DPAT by 28% and 30% in comparison to the oil-treated animals in the control viral-injected and ERβ-siRNA-Ads-injected animals, respectively (Figure 5A). The injection of ERβ-siRNA-Ads did not alter the effects of EB treatment on (+)8-OH-DPAT-stimulated oxytocin release in comparison to the control virus- injection. The three-way ANOVA for plasma oxytocin indicated a significant effect of (+)8-OH-DPAT injection (F(1, 40) = 220.85, p<0.0001), EB treatment (F(1, 40) = 10.386, p=0.0025), and a significant interaction between EB treatment and (+)8-OH-DPAT injection (F(1,40) = 6.986, p = 0.01). However, there was no significant effect of viral injection (F(1, 40) = 0.072, p= 0.79) and no significant interaction between the viral injection and EB treatment (F(1,40) = 0.191, p = 0.66), viral injection and (+)8-OH-DPAT injection (F(1,40) = 0.007, p = 0.93), or viral injection, EB treatment, and (+)8-OH-DPAT injection (F(1,40) = 0.045, p = 0.83).
Figure 5.
Effects of ERβ-siRNA-Ads on EB-induced alteration in the plasma oxytocin (A) and ACTH (B) response to (+)8-OH-DPAT. Data are presented as mean ± SEM (n=4–10). *: Significant difference from saline-challenged animals with same pre-treatment., #: significant difference from control virus/oil/(+)8-OH-DPAT injected animal, &: significant difference from ERβ-siRNA-Ads/oil/(+)8-OH-DPAT injected animals, @: significant difference from ERβ-siRNA-Ads/oil/(+)8-OH-DPAT injected, control virus/oil/(+)8-OH-DPAT injected, and control virus/EB/(+)8-OH-DPAT injected animals, (p<0.05) by Student-Newman-Keuls post hoc test.
Baseline levels of plasma ACTH were not different in control viral-injected or ERβ-siRNA-Ads-injected animals. In the control viral-injected animals, (+)8-OH-DPAT increased plasma ACTH levels. The effect of (+)8-OH-DPAT was decreased 23% by EB treatment (Figure 5B). In the ERβ-siRNA-Ads injected animals, the ACTH response to (+)8-OH-DPAT in oil-injected animals was 27% lower than that in the control virus/oil-injected animals (Figure 5B). In the ERβ-siRNA-Ads-injected animals, EB treatment resulted in a 43% increase in the plasma ACTH response to (+)8-OH-DPAT in comparison to the ERβ-siRNA-Ads/oil-injected animals (Figure 5B). The three-way ANOVA for plasma ACTH indicated a significant effect of EB treatment (F(1, 46) = 4.29, p < 0.05), (+)8-OH-DPAT injection (F(1, 46) = 390.18, p<0.0001), significant interaction between viral injection and EB treatment (F(1,46) = 22.41, p<0.0001), and significant interaction between the viral injection, EB treatment, and (+)8-OH-DPAT injection (F(1,46) = 20.11, p<0.0001). There was no significant effect of the viral injection (F(1, 46) = 2.1, p = 0.16), no significant interaction between the viral injection and (+)8-OH-DPAT injection (F(1,46) = 1.741, p = 0.19), or between the EB treatment and (+)8-OH-DPAT injection (F(1,46) = 3.11, p = 0.08).
Discussion
Previously, we reported that peripheral administration of EB or intra-PVN injections of G-1, a selective GPR30 agonist, for two days resulted in desensitization of 5-HT1A receptor signaling in the PVN (D'Souza et al., 2004; Raap et al., 2000; Xu et al., 2009). In the current study, we found that EB-mediated desensitization of the 5-HT1A receptor signaling in the PVN is independent of ERβ. However the effects of estradiol on ACTH release are more complex.
To determine whether ERβ is involved in the EB-induced desensitization of 5-HT1A receptor signaling in the PVN, we first examined the effects of ERβ agonist, DPN, on neuroendocrine responses to 5-HT1A receptor activation. Since treatment with GPR30 agonists result in desensitization of 5-HT1A receptor signaling (Xu et al., 2009) and no data were available concerning the affinity of DPN for GPR30, we determined the affinity of DPN for GPR30. We found that DPN has a 100 fold lower affinity for the GPR30 receptor in comparison to EB. Thus, DPN could be used to selectively stimulate ERβ. We found that DPN administration for two days did not alter the 5-HT1A receptor-stimulated release of oxytocin and ACTH. The dose of DPN (2 mg/kg) used in the current experiments is comparable to an effective dose used by other investigators in female rats to activate ERβ as demonstrated by decreases in anxiety behaviors, regulation of dopamine-2 receptors, and increases in cell proliferation (Le Saux et al., 2006; Lund et al., 2005; Mazzucco et al., 2006). Therefore, the present results suggest that activation of ERβ may be not involved in the desensitization of the 5-HT1A receptor signaling in the PVN. We used the same number of doses and timepoints for administration of DPN that we used for EB administration in order to determine if EB was working via ERβ. However, it is possible that other time points for administration of DPN would result in desensitization of 5-HT1A receptor signaling in the PVN.
To further evaluate the contribution of ERβ to the EB-mediated desensitization of the 5-HT1A receptor signaling, an adenovirus containing siRNA sequences against ERβ (ERβ siRNA-Ads) was directly injected into the PVN. Treatment with the ERβ siRNA-Ads decreased the protein levels of ERβ in the PVN by 34%. On the other hand, administration of EB for two days reduced the protein level of ERβ in the PVN by a comparable amount, 33%. This is consistent with the findings from other investigators who found that three to seven days of treatment with EB decreases ERβ mRNA and protein expression in the PVN (Patisaul et al., 1999; Suzuki and Handa, 2004). We found that EB administration regulates ERβ protein levels in the PVN to a similar extent as our ERβ siRNA-Ads injections. The combined treatment with both EB and ERβ siRNA-Ads resulted in a 50% decrease in the protein levels of ERβ in the PVN.
As we previously observed, two days of EB treatment desensitized 5-HT1A receptor signaling in oxytocin cells in the PVN (D'Souza et al., 2004; Raap et al., 2000). It appears that ERβ does not mediate the EB-induced desensitization of the 5-HT1A receptor signaling in oxytocin cells in the PVN based on the results from both experiments. First, treatment with the selective ERβ agonist DPN for two days did not desensitize 5-HT1A receptor signaling. Secondly, decreasing the ERβ levels in the PVN did not alter the effects of EB on 5-HT1A receptor agonist-stimulated release of oxytocin. The oxytocin neurons express very little ERα (Simonian and Herbison, 1997), thus this receptor is unlikely to contribute to the desensitization of the 5-HT1A receptors. However, studies to directly address the role of ERα in EB-mediated desensitization of 5-HT1A receptor signaling are needed to unequivocally resolve the question. Taken together with our previous findings that two days of treatment with the selective GPR30 agonist G-1 induces desensitization of 5-HT1A receptors (Xu et al., 2009), we propose that GPR30 mediates the EB-induced desensitization of the 5-HT1A receptor signaling in oxytocin cells.
EB-induced regulation of 5-HT1A receptor signaling in CRH-containing cells in the PVN, measured using ACTH as the peripheral marker for central activation of CRH neurons, is more complex. EB treatment has not been shown to consistently desensitize 5-HT1A receptor-mediated ACTH release (D'Souza et al., 2004; Raap et al., 2000). In the current experiment, desensitization of the 5-HT1A receptor signaling in CRH-containing cells was observed in rats treated with the control virus and EB. Based on our DPN data, it would appear that ERβ is not directly involved in the desensitization of the 5-HT1A receptor signaling in CRH cells. However, decreasing ERβ in the PVN differentially altered the ACTH response to 5-HT1A receptor activation in oil and EB treated rats. Recent reports suggest that estrogens and their receptors regulate CRH expression and release via complex mechanisms (Solomon and Herman, 2009). The ACTH response to 5-HT1A receptor activation in ERβ-siRNA-Ad injected rats may be mediated by different mechanisms in the absence and presence EB. We speculate that the decrease in ACTH release in the ERβ-siRNA-Ads/oil treated animals could be due to ERβ-mediated EB-independent regulation. Some splice variants of ERβ can function as transcription factors in the absence of ligand resulting in increased expression of several proteins including CRH (Miller et al., 2004) and CRH binding protein (van de Stolpe et al., 2004). Thus it would follow that decreasing ERβ expression with the ERβ-siRNA-Ads could decrease the transcription of CRH and reduce plasma ACTH levels. On the other hand, in the presence of EB, the activation of CRH neurons is regulated by both ERα and ERβ with opposite effects (Weiser and Handa, 2009). EB-induced activation of ERα in GABAergic neurons of the peri-PVN region impairs glucocorticoid-dependent negative feedback regulation of the HPA axis, resulting in an increase in ACTH release (Weiser and Handa, 2009). In contrast, activation of ERβ in the PVN slightly suppresses ACTH release (Weiser and Handa, 2009). Thus, the net effect of EB on HPA axis activity depends on the balance of ERα and ERβ actions. In ERβ-siRNA-Ad treated rats, the activity of ERβ is decreased, resulting in an unopposed action of ERα in response to EB treatment that may explain why the ACTH response to 8-OH-DPAT is potentiated in these rats. Further studies are needed to test these possible underlying mechanisms.
Several groups have shown that estrogen regulates 5-HT1A receptors in various regions of the brain, but the specific estrogen receptor subtypes involved have not been directly addressed as in this current study. We focused on the regulation of 5-HT1A receptors in the PVN by estrogen because this region is important in the regulation of stress and the control of mood (Carrasco and Van de Kar, 2003; Legros et al., 1993; Raadsheer et al., 1995). Since desensitization of pre-synaptic and post-synaptic 5-HT1A receptors may be an underlying mechanism by which SSRIs slowly mediate their therapeutic effects (Bosker et al., 2001; Chaput et al., 1986; Czachura and Rasmussen, 2000; Kreiss and Lucki, 1995), it is important to determine which estrogen receptors are capable of rapid desensitization of 5-HT1A receptor function. The current study suggests that in the PVN the 5-HT1A receptor desensitization is independent of ERβ. It was previously demonstrated that 1) 5-HT1A receptors in the PVN mediate the increased release of oxytocin and ACTH that is induced by peripheral injections of 8-OH-DPAT (Osei-Owasu et al., 2005) and 2) that the desensitization response induced by peripheral estradiol injections can be blocked by intra-PVN injections of pertussis toxin Xu et al., 2008). Further evidence for a direct effect of EB on desensitization of 5-HT1A receptors in the PVN comes from our previous study that demonstrated increased expression RGSz1 in the PVN with EB treatment (Carrasco et al., 2000, Neuroscience). An increase in RGSz1 can underlie the desensitization response since RGSz1 terminates Gz signaling and we have demonstrated that Gz is the Galpha protein that mediates 5-HT1A receptor stimulated release of oxytocin and ACTH (Serres et al., 2000, J Neuroscience). However, indirect effects of EB on 5-HT1A receptor signaling are possible as are direct effects of EB on oxytocin. Future studies would have to address whether or not ERβ regulates 5-HT1A receptors in other regions of the brain and also whether ERα is involved in 5-HT1A receptor regulation in the PVN and other brain regions. Determining which estrogen receptors mediate the EB-induced desensitization of 5-HT1A receptor signaling may allow us in the future to design treatments that activate specific estrogen receptor subtypes as an adjunct treatment with SSRIs for depression and other mood disorders.
Acknowledgments
The authors are grateful to Dr. Rakesh Singh and Cynthia M. Gouvion for technical assistance. The authors are also grateful to Dr. Tong-Chuan He from the University of Chicago for providing the constructs for the recombinant siRNA adenovirus and invaluable advice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alves SE, Lopez V, McEwen BS, Weiland NG. Differential colocalization of estrogen receptor beta (ERbeta) with oxytocin and vasopressin in the paraventricular and supraoptic nuclei of the female rat brain: an immunocytochemical study. Proc Natl Acad Sci U S A. 1998;95:3281–3286. doi: 10.1073/pnas.95.6.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpels JC. The female brain hypoestrogenic continuum from the premenstrual syndrome to menopause. A hypothesis and review of supporting data. J Reprod Med. 1996;41:633–639. [PubMed] [Google Scholar]
- Banger M. Affective syndrome during perimenopause. Maturitas. 2002;41 Suppl 1:S13–S18. doi: 10.1016/s0378-5122(02)00011-7. [DOI] [PubMed] [Google Scholar]
- Birzniece V, Johansson IM, Wang MD, Seckl JR, Backstrom T, Olsson T. Serotonin 5-HT(1A) receptor mRNA expression in dorsal hippocampus and raphe nuclei after gonadal hormone manipulation in female rats. Neuroendocrinology. 2001;74:135–142. doi: 10.1159/000054679. [DOI] [PubMed] [Google Scholar]
- Bosker FJ, Cremers TI, Jongsma ME, Westerink BH, Wikstrom HV, den Boer JA. Acute and chronic effects of citalopram on postsynaptic 5-hydroxytryptamine(1A) receptor-mediated feedback: a microdialysis study in the amygdala. J Neurochem. 2001;76:1645–1653. doi: 10.1046/j.1471-4159.2001.00194.x. [DOI] [PubMed] [Google Scholar]
- Carrasco GA, Van de Kar LD. Neuroendocrine pharmacology of stress. Eur J Pharmacol. 2003;463:235–272. doi: 10.1016/s0014-2999(03)01285-8. [DOI] [PubMed] [Google Scholar]
- Chaput Y, de Montigny C, Blier P. Effects of a selective 5-HT reuptake blocker, citalopram, on the sensitivity of 5-HT autoreceptors: electrophysiological studies in the rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1986;333:342–348. doi: 10.1007/BF00500007. [DOI] [PubMed] [Google Scholar]
- Critchley DJ, Childs KJ, Middlefell VC, Dourish CT. Inhibition of 8-OH-DPAT-induced elevation of plasma corticotrophin by the 5-HT1A receptor antagonist WAY100635. Eur J Pharmacol. 1994;264:95–97. doi: 10.1016/0014-2999(94)90642-4. [DOI] [PubMed] [Google Scholar]
- Czachura JF, Rasmussen K. Effects of acute and chronic administration of fluoxetine on the activity of serotonergic neurons in the dorsal raphe nucleus of the rat. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:266–275. doi: 10.1007/s002100000290. [DOI] [PubMed] [Google Scholar]
- D'Souza DN, Zhang Y, Damjanoska KJ, Carrasco GA, Sullivan NR, Garcia F, Battaglia G, Doncarlos LL, Muma NA, Van de Kar LD. Estrogen reduces serotonin-1A receptor-mediated oxytocin release and Galpha(i/o/z) proteins in the hypothalamus of ovariectomized rats. Neuroendocrinology. 2004;80:31–41. doi: 10.1159/000080795. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Gundlah C, Pecins-Thompson M, Schutzer WE, Bethea CL. Ovarian steroid effects on serotonin 1A, 2A and 2C receptor mRNA in macaque hypothalamus. Brain Res Mol Brain Res. 1999;63:325–339. doi: 10.1016/s0169-328x(98)00295-2. [DOI] [PubMed] [Google Scholar]
- Halbreich U. Gonadal hormones and antihormones, serotonin and mood. Psychopharmacol Bull. 1990;26:291–295. [PubMed] [Google Scholar]
- He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabovszky E, Kallo I, Steinhauser A, Merchenthaler I, Coen CW, Petersen SL, Liposits Z. Estrogen receptor-beta in oxytocin and vasopressin neurons of the rat and human hypothalamus: Immunocytochemical and in situ hybridization studies. J Comp Neurol. 2004;473:315–333. doi: 10.1002/cne.20127. [DOI] [PubMed] [Google Scholar]
- Jackson A, Uphouse L. Prior treatment with estrogen attenuates the effects of the 5-HT1A agonist, 8-OH-DPAT, on lordosis behavior. Horm Behav. 1996;30:145–152. doi: 10.1006/hbeh.1996.0018. [DOI] [PubMed] [Google Scholar]
- Jackson A, Uphouse L. Dose-dependent effects of estradiol benzoate on 5-HT1A receptor agonist action. Brain Res. 1998;796:299–302. doi: 10.1016/s0006-8993(98)00238-8. [DOI] [PubMed] [Google Scholar]
- Jimerson DC, Wolfe BE, Metzger ED, Finkelstein DM, Cooper TB, Levine JM. Decreased serotonin function in bulimia nervosa. Arch Gen Psychiatry. 1997;54:529–534. doi: 10.1001/archpsyc.1997.01830180043005. [DOI] [PubMed] [Google Scholar]
- Joffe H, Cohen LS. Estrogen, serotonin, and mood disturbance: where is the therapeutic bridge? Biol Psychiatry. 1998;44:798–811. doi: 10.1016/s0006-3223(98)00169-3. [DOI] [PubMed] [Google Scholar]
- Kreiss DS, Lucki I. Effects of acute and repeated administration of antidepressant drugs on extracellular levels of 5-hydroxytryptamine measured in vivo. J Pharmacol Exp Ther. 1995;274:866–876. [PubMed] [Google Scholar]
- Laflamme N, Nappi RE, Drolet G, Labrie C, Rivest S. Expression and neuropeptidergic characterization of estrogen receptors (ERalpha and ERbeta) throughout the rat brain: anatomical evidence of distinct roles of each subtype. J Neurobiol. 1998;36:357–378. doi: 10.1002/(sici)1097-4695(19980905)36:3<357::aid-neu5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Le Saux M, Di Paolo T. Changes in 5-HT1A receptor binding and G-protein activation in the rat brain after estrogen treatment: comparison with tamoxifen and raloxifene. J Psychiatry Neurosci. 2005;30:110–117. [PMC free article] [PubMed] [Google Scholar]
- Le Saux M, Morissette M, Di Paolo T. ERbeta mediates the estradiol increase of D2 receptors in rat striatum and nucleus accumbens. Neuropharmacology. 2006;50:451–457. doi: 10.1016/j.neuropharm.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Legros JJ, Ansseau M, Timsit-Berthier M. Neurohypophyseal peptides and psychiatric diseases. Regul Pept. 1993;45:133–138. doi: 10.1016/0167-0115(93)90195-e. [DOI] [PubMed] [Google Scholar]
- Lemonde S, Turecki G, Bakish D, Du L, Hrdina PD, Bown CD, Sequeira A, Kushwaha N, Morris SJ, Basak A, Ou XM, Albert PR. Impaired repression at a 5-hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide. J Neurosci. 2003;23:8788–8799. doi: 10.1523/JNEUROSCI.23-25-08788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy N, Paruthiyil S, Zhao X, Vivar OI, Saunier EF, Griffin C, Tagliaferri M, Cohen I, Speed TP, Leitman DC. Unliganded estrogen receptor-beta regulation of genes is inhibited by tamoxifen. Mol Cell Endocrinol. 2009 doi: 10.1016/j.mce.2009.08.030. [DOI] [PubMed] [Google Scholar]
- Li Q, Holmes A, Ma L, Van de Kar LD, Garcia F, Murphy DL. Medial hypothalamic 5-hydroxytryptamine (5-HT)1A receptors regulate neuroendocrine responses to stress and exploratory locomotor activity: application of recombinant adenovirus containing 5-HT1A sequences. J Neurosci. 2004;24:10868–10877. doi: 10.1523/JNEUROSCI.3223-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Levy AD, Cabrera TM, Brownfield MS, Battaglia G, Van de Kar LD. Long-term fluoxetine, but not desipramine, inhibits the ACTH and oxytocin responses to the 5-HT1A agonist, 8-OH-DPAT, in male rats. Brain Res. 1993;630:148–156. doi: 10.1016/0006-8993(93)90652-4. [DOI] [PubMed] [Google Scholar]
- Li Q, Muma NA, Battaglia G, Van de Kar LD. A desensitization of hypothalamic 5-HT1A receptors by repeated injections of paroxetine: reduction in the levels of G(i) and G(o) proteins and neuroendocrine responses, but not in the density of 5-HT1A receptors. J Pharmacol Exp Ther. 1997;282:1581–1590. [PubMed] [Google Scholar]
- Li Q, Muma NA, van de Kar LD. Chronic fluoxetine induces a gradual desensitization of 5-HT1A receptors: reductions in hypothalamic and midbrain Gi and G(o) proteins and in neuroendocrine responses to a 5-HT1A agonist. J Pharmacol Exp Ther. 1996;279:1035–1042. [PubMed] [Google Scholar]
- Lu NZ, Bethea CL. Ovarian steroid regulation of 5-HT1A receptor binding and G protein activation in female monkeys. Neuropsychopharmacology. 2002;27:12–24. doi: 10.1016/S0893-133X(01)00423-7. [DOI] [PubMed] [Google Scholar]
- Lund TD, Rovis T, Chung WC, Handa RJ. Novel actions of estrogen receptor-beta on anxiety-related behaviors. Endocrinology. 2005;146:797–807. doi: 10.1210/en.2004-1158. [DOI] [PubMed] [Google Scholar]
- Luo J, Deng ZL, Luo X, Tang N, Song WX, Chen J, Sharff KA, Luu HH, Haydon RC, Kinzler KW, Vogelstein B, He TC. A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nat Protoc. 2007;2:1236–1247. doi: 10.1038/nprot.2007.135. [DOI] [PubMed] [Google Scholar]
- Mazzucco CA, Lieblich SE, Bingham BI, Williamson MA, Viau V, Galea LA. Both estrogen receptor alpha and estrogen receptor beta agonists enhance cell proliferation in the dentate gyrus of adult female rats. Neuroscience. 2006;141:1793–1800. doi: 10.1016/j.neuroscience.2006.05.032. [DOI] [PubMed] [Google Scholar]
- Menkes DB, Coates DC, Fawcett JP. Acute tryptophan depletion aggravates premenstrual syndrome. J Affect Disord. 1994;32:37–44. doi: 10.1016/0165-0327(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Miller WJ, Suzuki S, Miller LK, Handa R, Uht RM. Estrogen receptor (ER)beta isoforms rather than ERalpha regulate corticotropin-releasing hormone promoter activity through an alternate pathway. J Neurosci. 2004;24:10628–10635. doi: 10.1523/JNEUROSCI.5540-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mize AL, Alper RH. Acute and long-term effects of 17beta-estradiol on G(i/o) coupled neurotransmitter receptor function in the female rat brain as assessed by agonist-stimulated [35S]GTPgammaS binding. Brain Res. 2000;859:326–333. doi: 10.1016/s0006-8993(00)01998-3. [DOI] [PubMed] [Google Scholar]
- Osei-Owusu P, James A, Crane J, Scrogin KE. 5-Hydroxytryptamine 1A receptors in the paraventricular nucleus of the hypothalamus mediate oxytocin and adrenocorticotropin hormone release and some behavioral components of the serotonin syndrome. J Pharmacol Exp Ther. 2005;313:1324–1330. doi: 10.1124/jpet.104.082073. [DOI] [PubMed] [Google Scholar]
- Osterlund MK, Hurd YL. Acute 17 beta-estradiol treatment down-regulates serotonin 5HT1A receptor mRNA expression in the limbic system of female rats. Brain Res Mol Brain Res. 1998;55:169–172. doi: 10.1016/s0169-328x(98)00018-7. [DOI] [PubMed] [Google Scholar]
- Pang Y, Dong J, Thomas P. Estrogen signaling characteristics of Atlantic croaker G protein-coupled receptor 30 (GPR30) and evidence it is involved in maintenance of oocyte meiotic arrest. Endocrinology. 2008;149:3410–3426. doi: 10.1210/en.2007-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patisaul HB, Whitten PL, Young LJ. Regulation of estrogen receptor beta mRNA in the brain: opposite effects of 17beta-estradiol and the phytoestrogen, coumestrol. Brain Res Mol Brain Res. 1999;67:165–171. doi: 10.1016/s0169-328x(99)00058-3. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6th ed. New York: Academic Press; 2007. [Google Scholar]
- Pecins-Thompson M, Bethea CL. Ovarian steroid regulation of serotonin-1A autoreceptor messenger RNA expression in the dorsal raphe of rhesus macaques. Neuroscience. 1999;89:267–277. doi: 10.1016/s0306-4522(98)00326-1. [DOI] [PubMed] [Google Scholar]
- Raadsheer FC, van Heerikhuize JJ, Lucassen PJ, Hoogendijk WJ, Tilders FJ, Swaab DF. Corticotropin-releasing hormone mRNA levels in the paraventricular nucleus of patients with Alzheimer's disease and depression. Am J Psychiatry. 1995;152:1372–1376. doi: 10.1176/ajp.152.9.1372. [DOI] [PubMed] [Google Scholar]
- Raap DK, DonCarlos L, Garcia F, Muma NA, Wolf WA, Battaglia G, Van de Kar LD. Estrogen desensitizes 5-HT(1A) receptors and reduces levels of G(z), G(i1) and G(i3) proteins in the hypothalamus. Neuropharmacology. 2000;39:1823–1832. doi: 10.1016/s0028-3908(99)00264-6. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety. 2000;12 Suppl 1:2–19. doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Savitz J, Lucki I, Drevets WC. 5-HT(1A) receptor function in major depressive disorder. Prog Neurobiol. 2009;88:17–31. doi: 10.1016/j.pneurobio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider LS, Small GW, Hamilton SH, Bystritsky A, Nemeroff CB, Meyers BS Fluoxetine Collaborative Study Group. Estrogen replacement and response to fluoxetine in a multicenter geriatric depression trial. Am J Geriatr Psychiatry. 1997;5:97–106. [PubMed] [Google Scholar]
- Shively CA, Bethea CL. Cognition, mood disorders, and sex hormones. ILAR J. 2004;45:189–199. doi: 10.1093/ilar.45.2.189. [DOI] [PubMed] [Google Scholar]
- Simonian SX, Herbison AE. Differential expression of estrogen receptor alpha and beta immunoreactivity by oxytocin neurons of rat paraventricular nucleus. J Neuroendocrinol. 1997;9:803–806. doi: 10.1046/j.1365-2826.1997.00659.x. [DOI] [PubMed] [Google Scholar]
- Solomon MB, Herman JP. Sex differences in psychopathology: Of gonads, adrenals and mental illness. Physiol Behav. 2009 doi: 10.1016/j.physbeh.2009.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Handa RJ. Regulation of estrogen receptor-beta expression in the female rat hypothalamus: differential effects of dexamethasone and estradiol. Endocrinology. 2004;145:3658–3670. doi: 10.1210/en.2003-1688. [DOI] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146:624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- van de Stolpe A, Slycke AJ, Reinders MO, Zomer AW, Goodenough S, Behl C, Seasholtz AF, van der Saag PT. Estrogen receptor (ER)-mediated transcriptional regulation of the human corticotropin-releasing hormone-binding protein promoter: differential effects of ERalpha and ERbeta. Mol Endocrinol. 2004;18:2908–2923. doi: 10.1210/me.2003-0446. [DOI] [PubMed] [Google Scholar]
- Vicentic A, Li Q, Battaglia G, Van de Kar LD. WAY-100635 inhibits 8-OH-DPAT-stimulated oxytocin, ACTH and corticosterone, but not prolactin secretion. Eur J Pharmacol. 1998;346:261–266. doi: 10.1016/s0014-2999(97)01607-5. [DOI] [PubMed] [Google Scholar]
- Weiser MJ, Handa RJ. Estrogen impairs glucocorticoid dependent negative feedback on the hypothalamic-pituitary-adrenal axis via estrogen receptor alpha within the hypothalamus. Neuroscience. 2009;159:883–895. doi: 10.1016/j.neuroscience.2008.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Qin S, Carrasco GA, Dai Y, Filardo EJ, Prossnitz ER, Battaglia G, Doncarlos LL, Muma NA. Extra-nuclear estrogen receptor GPR30 regulates serotonin function in rat hypothalamus. Neuroscience. 2009;158:1599–1607. doi: 10.1016/j.neuroscience.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]