Figure 6.
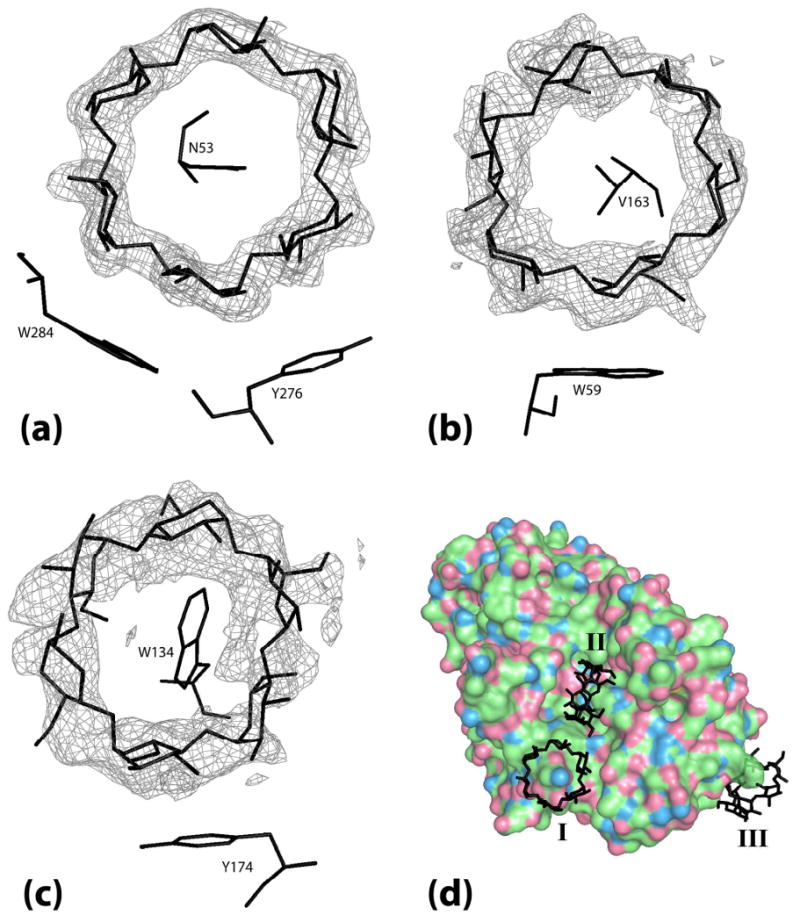
The electron density for each α-cyclodextrin ring is from a Fo-Fc map phased by the final model with the α-cyclodextrin ring that the density covers removed. (a) α-cyclodextrin I bridging between two PPA molecules superimposed on the map density contoured at 4.0 σ. (b) α-cyclodextrin II bound in the binding site cleft superimposed on map density contoured at 2.0 σ. (c) α-cyclodextrin III bound at Trp134 superimposed on map density contoured at 1.8 σ. (d) Full view of the 3 molecules of α-cyclodextrin bound on the surface of PPA with α-cyclodextrins labeled I, II, and III.
