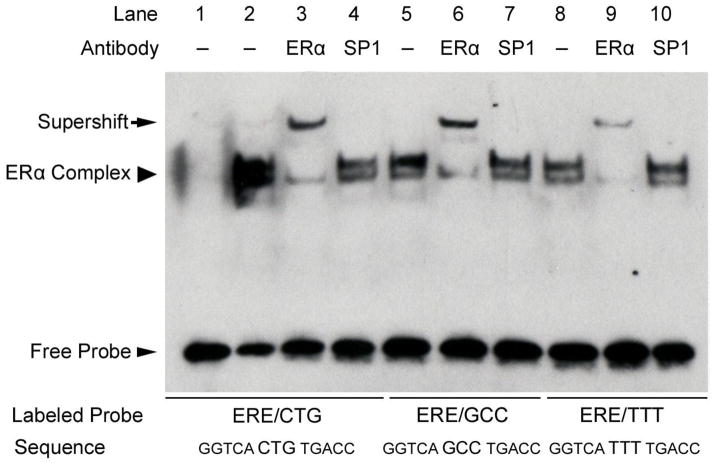
Fig. 1.
The tri-nucleotide spacer sequence modulates ERα-ERE binding affinity. EMSA of ERα binding to consensus ERE sequences with variable tri-nucleotide spacer sequences. An ERα-containing complex bound to all three ERE sequences (arrowhead, lanes 2, 5, and 8) and was confirmed by supershift (arrow) using a monoclonal antibody that recognizes ERα (lanes 3, 6, and 9). Receptor binding affinity for the sequences favored tri-nucleotide spacer sequences of CTG > GCC > TTT. The non-specific antibody recognizing Sp1 had no effect on the ERα-containing complexes bound to these probes (lanes 4, 7, and 10). Shown is a representative experiment performed at least three times.