Abstract
Mitochondria are dynamic organelles that constantly undergo fission, fusion, and movement. Increasing evidence indicates that these dynamic changes are intricately related to mitochondrial function, suggesting that mitochondrial form and function are linked. Calcium (Ca2+) is one signal that has been shown to both regulate mitochondrial fission in various cells types and stimulate mitochondrial enzymes involved in ATP generation. However, although Ca2+ plays an important role in the adult cardiac muscle cells for excitation-metabolism coupling, little is known about whether Ca2+ can regulate their mitochondrial morphology. Therefore, we tested the role of Ca2+ in regulating cardiac mitochondrial fission. We found that neonatal and adult cardiomyocyte mitochondria undergo rapid and transient fragmentation upon a thapsigargin (TG)- or KCl-induced cytosolic Ca2+ increase. The mitochondrial fission protein, DLP1, participates in this mitochondrial fragmentation, suggesting that cardiac mitochondrial fission machinery may be regulated by intracellular Ca2+ signaling. Moreover, the TG-induced fragmentation was also associated with an increase in reactive oxygen species (ROS) formation, suggesting that activation of mitochondrial fission machinery is an early event for Ca2+-mediated ROS generation in cardiac myocytes. These results suggest that Ca2+, an important regulator of muscle contraction and energy generation, also dynamically regulates mitochondrial morphology and ROS generation in cardiac myocytes.
Keywords: Mitochondria, mitochondrial fusion and fission, calcium, ventricular myocytes, reactive oxygen species, cardiac muscle
1. INTRODUCTION
Cardiac myocytes, with their high metabolic demands, are comprised of approximately 35% mitochondrial volume per cell [1]. These mitochondria, mostly situated among the contractile filaments and next to the sarcoplasmic reticulum (SR), perform the important role of providing ATP and regulating Ca2+ in microdomains [2]. Mitochondrial shape varies greatly within the adult cardiomyocyte. Based on electron microscopic observations, interfibrillar mitochondria are relatively large and oval shaped and evenly distributed in a crystal-like pattern along the contractile apparatus, whereas the subsarcolemmal and perinuclear mitochondria vary in size and shape, and appear less organized [1].
Despite the variations in mitochondrial size and shape in individual myocytes, very little is known about mitochondrial dynamics in the adult heart. Several recent studies show the presence of mitochondrial fission and fusion proteins in heart tissue, indicating that mitochondrial structure may be dynamic in the heart as well [3–7]. In addition, the high incidence of abnormal mitochondrial morphologies associated with many cardiac diseases suggests a role for these processes in normal cardiac function and abnormal disease etiology [8–14]. However, due to the highly organized and more static structure of the mitochondria in adult cardiac myocyte, it remains unclear whether these mitochondria are capable of undergoing fusion and fission and if mitochondrial dynamics plays a physiological role in the heart.
In many cell types, the dynamic interconversion between mitochondrial fusion and fission is important for normal cellular function. This becomes apparent as mutations or knockouts of the genes involved in mitochondrial dynamics have been shown to be lethal as well as cause human diseases [15–22]. Each process requires a different cohort of proteins. For example, mitochondrial fission requires the 17kD mitochondrial outer membrane protein, hFis1, and the recruitment of the 80–85kD large cytosolic GTPase DLP1 to the mitochondrial surface via transient interaction with hFis1 [23, 24]. DLP1 is presumed to self-assemble into a homo-oligomeric ring around the mitochondrion [25] and possibly acts as a mechanoenzyme to pinch the mitochondrial membranes. GTP hydrolysis is required for the DLP1 constriction and disassembly, and a point mutation in this domain, such as DLP1-K38A, leads to a dominant negative fission-defective mutant and elongation of mitochondria due to reduced fission [26–29]. DLP1-K38A also has other effects on cellular physiology, such as changing the distribution and morphology of the endoplasmic reticulum (ER) [27].
In this study, we addressed the question of mitochondrial dynamics in the cardiac myocyte using thapsigargin (TG), a sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA) inhibitor, which has been previously shown to increase intracellular Ca2+ leading to mitochondrial fission via a DLP1-dependent process [30, 31]. We demonstrated that not only does Ca2+ regulate a DLP1-dependent mitochondrial fission but also ROS production. These findings are the first evidence to indicate the role of Ca2+ regulation of mitochondrial fragmentation and ROS generation in cardiac muscle cells.
2. MATERIALS AND METHODS
2.1. Neonatal rat ventricular cardiomyocyte culture
As modified from a previously published procedure [32], the hearts from 1–3 day old anesthetized Sprague-Dawley rats were aeseptically removed and transferred to HBSS containing 0.1% BSA and rinsed. Ventricles were minced and digested in BSA-free HBSS, 0.5mg/mL collagenase (Worthington, type II) solution at 37°C. The cells were filtered, pelleted, and resuspended in DMEM with 50U/mL penicillin G, 10% calf serum, and 10% horse serum. Cells were pre-plated once for 30 min and again for 60 min. Cells unattached to the plate were collected and plated on glass coverslips. After 24 hours the cultures were treated with 1:100 arabinosylcytosine (ara-C) for 48 hours to reduce the growth of fibroblasts. Cells were treated with fresh medium and used for experiments from day 5–7 in culture. In some cases, cells were transfected with a vector expressing either untagged or GFP-tagged mutant DLP1-K38A using FuGENE (Roche) according to manufacturer’s instructions as described previously [27]. For treatments and visualization, untransfected (control) and transfected (GFP positive) cells were examined from the same specimen.
2.2. Isolation of adult rat ventricular myocytes
The procedure was described in a published paper [33].
2.3. Ca2+ imaging
Modified from the procedure published previously [34], cells were loaded with 7 µM Fura-2/AM and 0.02% Pluronic F-127 (Invitrogen) in HEPES buffer (10 mM HEPES, 10 mM glucose, 140 mM NaCl, 5 mM KCl, 1.8 mM CaCl2, 2 mM MgCl2, pH 7.4) at 37°C for 30 min to allow the dye to accumulate in the cytosol. Cells were washed for 30 min at 37°C to allow for de-esterification before experiments. After TG (Sigma-Aldrich, Co) addition, cells were sequentially excited at 340 nm and 380 nm, and cytosolic free Ca2+ was detected at 510 nm. Mitochondrial Ca2+ was measured by loading cells with 5 µM Rhod-2/AM (Invitrogen) at 4°C for 15 min to allow the dye to accumulate in the mitochondria, for 15 min at room temperature, and were washed for 15 min. Cells were incubated with 10 µM Ru360 for 30 min at room temperature to block mitochondrial Ca2+ uptake. All graphs were plotted as F/F0 +/− SEM.
2.4. Fluorescence Imaging
Indirect immunofluorescence was performed as described previously [29, 35]. Cells were fixed with 4% paraformaldehyde, permeabilized, and incubated in blocking buffer containing 5% horse serum for 1 hour at 37°C. Rabbit anti-DLP-N (described previously [36]) or mouse monoclonal anti-cytochrome c antibodies (Pharmingen), and Alexa 488 or 594-conjugated antibodies (Invitrogen) were used for primary and secondary antibodies, respectively. After appropriate washing, coverslips were mounted in ProLong antifade reagent (Invitrogen) on glass slides.
Live mitochondria were visualized by either loading with 200 nM Mitotracker Red CMXRos, or 5 µM MitoSOX Red (Invitrogen) in cultured neonatal ventricular myocytes at 37°C in HEPES buffer. With a 150W xenon lamp from the illumination unit polychrome [36]V, single cell images were taken by fluorescent microscopy (TILL Photonics LLC, Pleasanton, CA). The fluorescent imaging system uses a Nikon TE2000s inverted microscope with a 40× oil objective. Neonatal ventricular myocyte mitochondria were analyzed as described previously [31, 37].
2.5. Mitochondrial Isolation
Cardiomyocytes were collected at 700 × g for 5 min at 4°C before and at various times after treatment with TG. The supernatant was discarded and the cell pellet was homogenized in 1 ml isolation buffer (10 mM HEPES pH 7.4, 1 mM EDTA, 320 mM sucrose, protease inhibitor). The homogenate was centrifuged at 700 × g for 8 min at 4°C and the supernatant was collected. The pellet was subjected to re-homogenization, centrifuged, and the supernatants were pooled. The supernatants were centrifuged at 17,000 × g for 11 min at 4°C and the supernatant (cytosol) and the pellet (mitochondria) were separated.
2.6. SDS-PAGE and Western Blotting
SDS-PAGE [38] and Western blotting was performed as described previously [36]. We used anti-DLP1-N [39], anti-Fis1 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-GAPDH (Millipore, Billerica, MA), and anti-VDAC (Calbiochem, San Diego, CA) antibodies. Blots were developed with supersignal chemiluminescence detection substrate (Pierce, Rockford, IL).
2.7. Electron Microscopy
Specimens were prepared for and analyzed by electron microscopy at the University of Rochester Core Facility, as described previously [40]. Mitochondria from adult ventricular myocytes were analyzed using AnalySIS software (University of Rochester, Electron Microscope Core Facility) for form factor, aspect ratio, and area.
2.8. Statistical analysis
The data were statistically analyzed by parametric (two tailed t-test, one way analysis of variance with Dunnett’s post-hoc testing) and non-parametric methods (Kruskal-Wallis with Dunn’s post-hoc testing) using either Prism (GraphPad Software, La Jolla, CA) or SPSS (SPSS Inc., Chicago, IL). A P value < 0.05 was considered statistically significant.
2.9.Ethical Aspects
Due to the lack of appropriate cell lines for cardiomyocytes, it was necessary to isolate cardiac cells in primary culture from newborn rats and adult rats. The animals were kept in the University of Rochester vivarium and treated humanely. Each animal was sacrificed consistent with UCAR protocol 2006063 and the Panel of Euthanasia of the American Veterinary Medical Association.
3. RESULTS
Both adult and neonatal rat ventricular myocytes were used for experiments. Cultured neonatal rat ventricular myocytes were used for live cell imaging of mitochondrial morphology and function, taking advantage of their thin morphology with a lower density of mitochondria and relative ease of transfection. In contrast, in order to evaluate mitochondrial morphology in adult ventricular myocytes, electron microscopy was used because their thicker cell diameter with densely populated mitochondria limited the spatial resolution of optical microscopy.
3.1. Mitochondrial fission proteins are expressed in rat ventricular myocytes and respond to elevated Ca2+ concentrations
Mitochondrial fission requires the two proteins, hFis1 and DLP1. Western blotting showed that both hFis1 and DLP1 were expressed in myocytes at day 2 and 7 in culture (Fig. 1A). This indicates that cardiac mitochondria possess the components necessary to undergo fission events, in agreement with the previous identification of DLP1 mRNA in adult human heart [6].
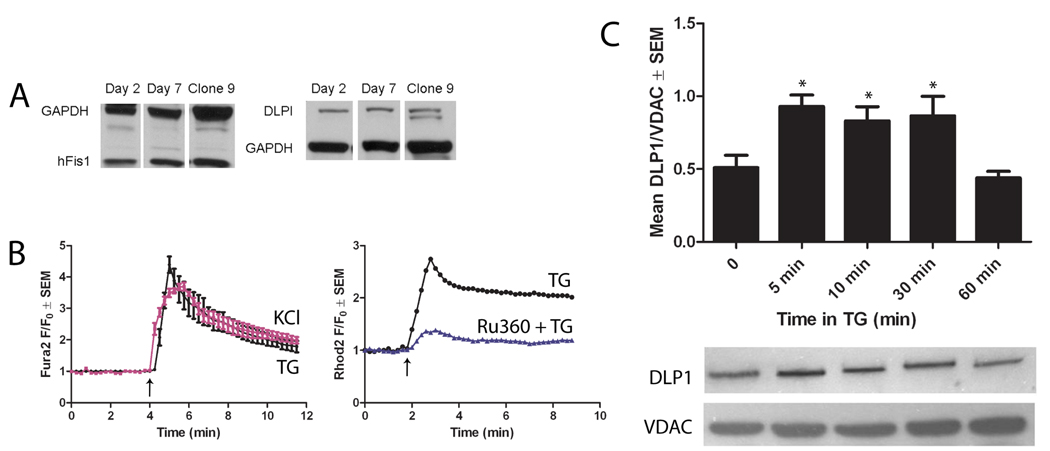
Figure 1. Ventricular myocytes express DLP1, which translocates to mitochondria upon TG-induced increase of cytosolic Ca2+.
(A) Western blotting demonstrates that neonatal rat ventricular myocytes endogenously express hFis1 and DLP1 from days 2–7 in culture. Clone 9 cells were used as a positive control for fission protein expression [23]. GAPDH was used as a loading control. (B) Ca2+ imaging using Fura-2 showed that either 1 µM TG (black line) or 50 mM KCl (pink line) induced an increase in cytosolic Ca2+. The Fura-2 ratio (F340/F360) at each time point (F) was normalized against the initial ratio (F0) for comparison (n=4). Representative traces of mitochondrial Ca2+ imaging using Rhod-2 showed that 1 µM TG (black line) induced mitochondrial Ca2+ uptake. Treatment with Ru360 (blue line) decreased mitochondrial Ca2+ uptake upon TG addition. (n=3) (C) Cultured adult cardiomyocytes were treated with 1 µM TG for 5, 10, 30, or 60 min and the mitochondrial fractions were isolated and assayed for DLP1 and VDAC (loading control) by Western blot. DLP1 association with mitochondria increased after 5, 10, and 30 min of TG treatment but returned to basal levels at 60 min TG (* P<0.01 by ANOVA with Dunnett’s post-hoc test, n=4).
TG is known to cause a global increase of cytosolic Ca2+ concentrations by inhibiting Ca2+ uptake at the SR/ER via SERCA [41]. In addition, TG has been shown to cause mitochondrial fragmentation via enhanced mitochondrial Ca2+ uptake [31]. Therefore, we used TG to examine whether mitochondria are able to undergo Ca2+ induced fragmentation in cardiac myocytes. Ca2+ imaging using Fura-2 showed that addition of 1 µM TG to cultured neonatal rat ventricular myocytes induced an immediate increase of cytosolic Ca2+ which gradually decreased to approximately half the peak by 10 min (Fig. 1B, left). Similar effects on cytosolic Ca2+ were observed after treatment with 50 mM KCl, which opens L-type Ca2+ channels via membrane depolarization (Fig. 1B, left). Mitochondrial Ca2+ imaging using Rhod-2 demonstrated that TG also induced a rise in mitochondrial free Ca2+, which could be blocked by preincubation with 10 µM Ru360, which inhibits mitochondrial Ca2+ uptake (Fig. 1B, right). Similar effects of TG were observed in adult rat ventricular myocytes (data not shown).
DLP1 translocation from the cytosol to mitochondria is an early step in mitochondrial fission [15, 16, 26]. We next assessed whether TG induced DLP1 translocation to mitochondria in isolated adult cardiac myocytes. This preparation provides a larger quantity of proteins than neonatal heart cells for such experiments. Freshly isolated adult rat cardiac myocytes were treated with 1 µM TG for 5, 10, 30, and 60 min, and the mitochondrial fraction was collected and examined for the presence of DLP1 by Western blot. TG caused an accumulation of DLP1 in the mitochondrial fraction at 5, 10, and 30 min compared to controls (Fig. 1C). However, after 60 min of treatment with TG, DLP1 returned to similar levels as controls, indicating that the TG-induced DLP1 translocation was a transient event (Fig. 1C). The time course of elevated Ca2+ levels and DLP1 accumulation in the mitochondrial fraction was similar suggesting that TG induced DLP1 accumulation via elevated Ca2+ levels. These results indicate that increased cytosolic Ca2+ in adult cardiac myocytes induces DLP1 translocation to mitochondria, an early event of mitochondrial fission. We obtained similar results treating cells with 50 mM KCl (data not shown).
3.2. TG induces a rapid change in mitochondrial morphology in neonatal and adult cardiac myocytes
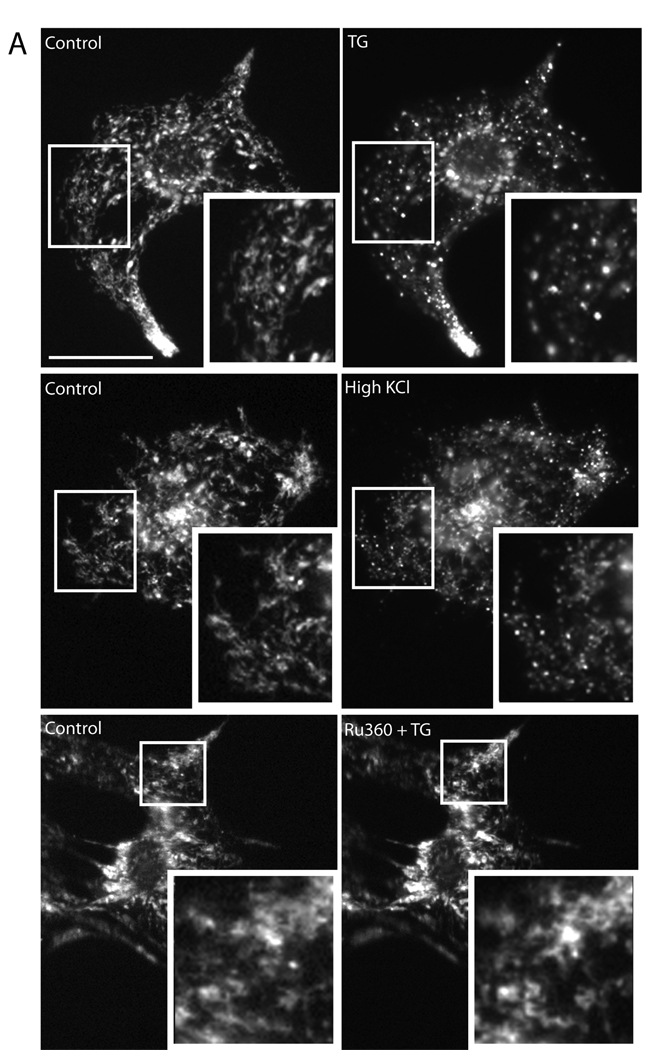
Next, we tested the effects of TG-induced cytosolic Ca2+ increase on mitochondrial morphology in rat neonatal ventricular myocytes. These myocytes normally displayed filamentous or tubular mitochondria along with large, globular mitochondria that were often continuous with mitochondrial tubules. We observed a rapid change in mitochondrial morphology upon invoking an increase in cytosolic Ca2+ with either 1 µM TG or 50 mM KCl. While mitochondria from untreated cells displayed normal tubular/globular morphology (Fig. 2A, control), within 5 min after the addition of TG or KCl, the tubular and globular mitochondria became small and punctate, indicating mitochondrial fragmentation (Fig. 2A and 3C). Furthermore, inhibition of mitochondrial Ca2+ uptake with Ru360 prevented mitochondrial fragmentation in TG-treated cells, suggesting that mitochondrial Ca2+ uptake is necessary for mitochondrial fragmentation (Fig. 2A).
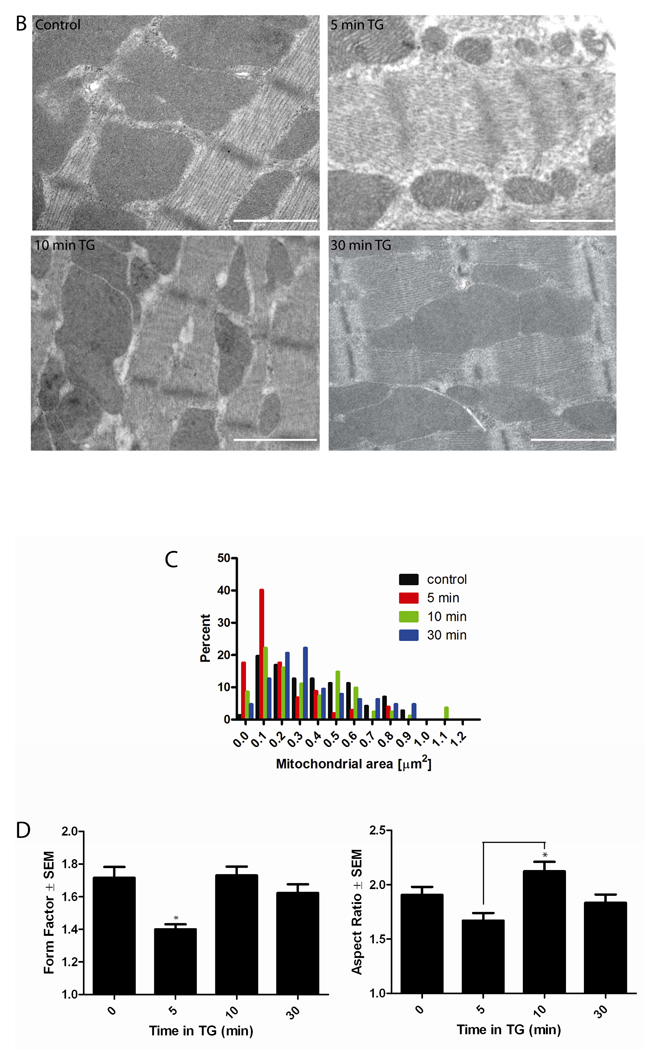
Figure 2. TG fragments mitochondria in neonatal and adult ventricular myocytes.
(A) Within 5 min, either 1 µM TG or 50 mM KCl, induced mitochondrial fragmentation, compared to baseline, in cultured neonatal myocytes. Untreated cells displayed normal globular and tubular mitochondrial morphology (A, control). Preincubation with 10 µM Ru360 for 30 min and throughout the experiment inhibited TG-induced mitochondrial fragmentation at 5 min. Mitochondria were visualized using Mitotracker Red CMXRos. Scale bar = 30 µm. (B) Cultured adult cardiac myocytes were treated with 1 µM TG for 5, 10, and 30 min, fixed, and viewed under electron microscope. Cardiac mitochondria display various morphologies in control and at each time point but mitochondria appeared smaller and more fragmented at 5 min TG. Scale bar = 1 µm. (C) Consistent with this observation, a histogram of mitochondrial area demonstrated a smaller mean mitochondrial area at 5 min TG, which recovered at 10 and 30 min TG compared to control. (D) Analysis of mitochondrial form factor demonstrates a decrease in mitochondrial branching and length from cells after 5 min of TG treatment compared to 0, 10, and 30 min TG, and a recovery of normal morphology at 10 and 30 min TG. Analysis of mitochondrial aspect ratio demonstrates shorter mitochondria at 5 min TG, with significant lengthening between 5 and 10 min followed by a return to baseline. (for all analyses, *P<0.05 using Kruskal-Wallis non-parametric testing with Dunn’s post-hoc test)
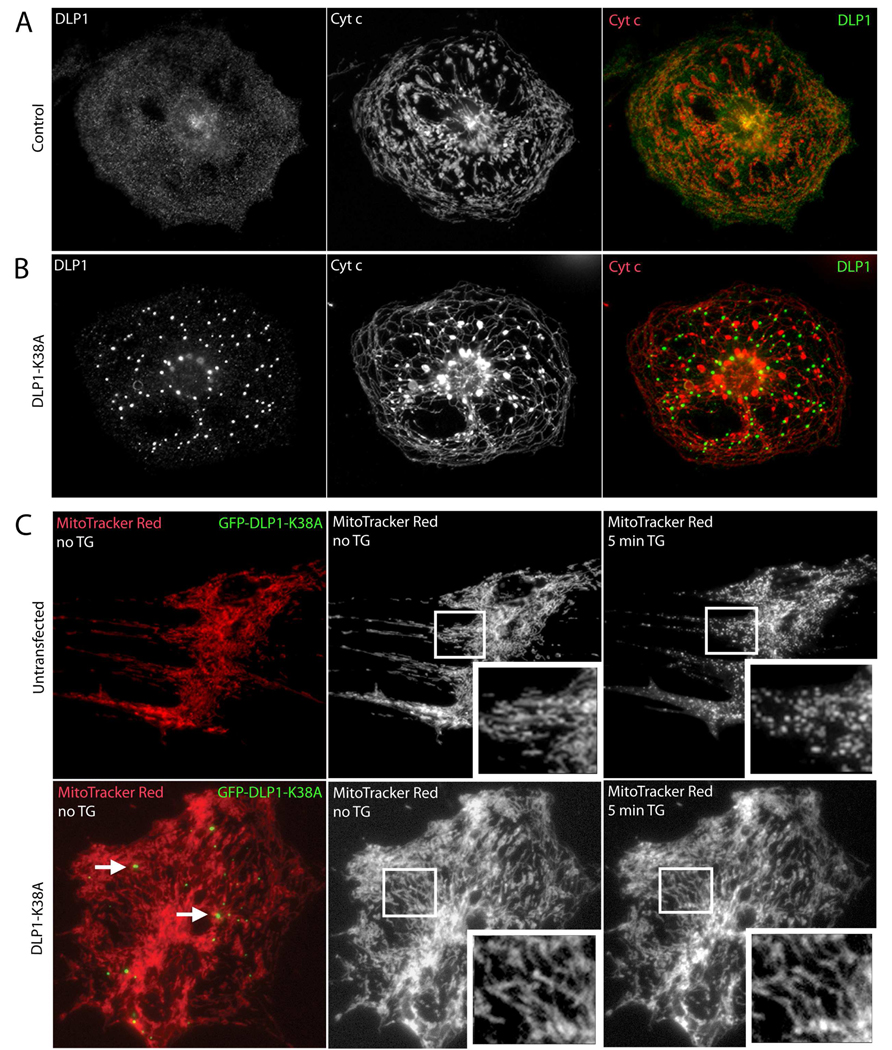
Figure 3. TG-induced mitochondrial fragmentation requires the mitochondrial fission protein DLP1 in neonatal ventricular myocytes.
(A) A control neonatal ventricular myocyte labeled with anti-DLP1 and anti-cytochrome c antibodies displays diffuse cytosolic DLP1 and a mix of globular and tubular mitochondria, while the overlay shows little association of DLP1 (green) with mitochondria (red). (B) A neonatal ventricular myocyte overexpressing the dominant negative fission mutant, DLP1-K38A, displays large cytosolic aggregates of DLP1, characteristic of mutant DLP1-K38A expression, and elongated and entangled mitochondria [27, 29]. The overlay shows that mutant DLP1 aggregates (green) do not colocalize with mitochondria (red). (C) Cells were transfected with GFP-tagged DLP1-K38A and live mitochondria were visualized using MitoTracker Red CMXRos. The cells that were positive for DLP1-K38A expression were identified by the presence of green mutant DLP1 aggregates (arrows, lower left), while untransfected cells did not display green aggregates (upper left). The mitochondrial network in control, untransfected cells became fragmented upon 5 min TG treatment (top), while TG has no effect on the elongated and tubular mitochondria seen with GFP-tagged DLP1-K38A overexpression (bottom).
To test if mitochondrial fragmentation could occur in adult cardiac myocytes, freshly isolated adult rat ventricular myocytes were treated with 1 µM TG for 5, 10, or 30 min, and immediately fixed and prepared for electron microscopy and morphometric analysis (Fig. 2B and C). We measured area, perimeter, and major and minor axes for individual mitochondria within single cells (2 experiments, 10 cells per time point). Analysis of mitochondrial area revealed that control cardiac muscle contained a wide range of mitochondrial sizes (mean = 0.372 µm2, range 0.039 to 0.873 µm2) with a non-Gaussian distribution. Therefore, a non-parametric analysis of area was performed. At 5 min TG treatment, mitochondrial area was significantly smaller than controls (mean = 0.189 µm2, range 0.007 to 0.833 µm2). At 10 and 30 min TG treatment, mitochondrial area had largely recovered to control values, although the range of areas remained wider (10 min: mean = 0.324 µm2, range 0.016 to 1.149 µm2 ; 30 min: mean = 0.360 µm2, range 0.020 to 0.892 µm2). A histogram of the frequency distribution of the area demonstrates these changes in mitochondrial area and suggests that TG induces an immediate (5 min) fragmentation of most mitochondria (Fig. 2C). At 10 and 30 min, mitochondria appeared to be recovering from this fragmentation.
We also measured aspect ratio (AR; major axis/minor axis), which is a measure of mitochondrial length, and form factor (FF; perimeter2/4π·Area), which is a measure of mitochondrial length and branching [37]. AR and FF both have a minimum value of 1 when the mitochondrion is a small, perfect circle, and these values increase as the mitochondrion becomes elongated and/or branched. During the time course of TG treatment, the FF was significantly decreased at 5 min compared to 0, 10, and 30 min TG, indicating that the mitochondria tended to be more fragmented and less branched (Fig. 2D). The values of FF at 10 and 30 min TG were similar to 0 min, indicating recovery of mitochondrial morphology. Analysis of AR revealed a trend toward shorter mitochondria after 5 min TG that was not statistically significant. However, at 10 min TG, mitochondria were significantly longer compared to 5 min TG, indicating that as the mitochondrial morphology recovered after fragmentation (Fig. 2D).
3.3. TG-induced mitochondrial fragmentation in cardiac myocytes requires the mitochondrial fission protein DLP1
To test whether TG induces the normal mitochondrial fission process, neonatal rat ventricular myocytes were transfected with dominant negative DLP1-K38A, and mitochondrial morphology was assessed before and after TG treatment. Control cells showed a cytoplasmic distribution of DLP1 and a mixture of elongated and large globular mitochondria with only a slight colocalization between the DLP1 and mitochondria (Fig. 3A). In contrast, overexpression of DLP1-K38A (detected with anti-DLP1 antibodies) caused the elongation of mitochondrial tubules that formed an interconnected network. Also, characteristic of dominant negative DLP1-K38A overexpression were the accumulations of large cytosolic DLP1 aggregates, which has been reported in previous studies [26–29, 31, 35] (Fig. 3B). Little colocalization between DLP1 and mitochondria was observed in the DLP1-K38A overexpressing cells (Fig. 3B).
When GFP-tagged DLP1-K38A overexpressing cells were treated with TG, the mitochondria in these cells remained elongated tubules, whereas untransfected (UT) cells from the same dish underwent drastic fragmentation of mitochondria after 5 min (Fig. 3C). Therefore, mutant DLP1 disrupted normal mitochondrial fission in neonatal myocytes and prevented TG-induced mitochondrial fragmentation. These data demonstrate that increased cytosolic Ca2+ may act upstream of the DLP1 scission enzyme to activate the cellular mitochondrial fission machinery.
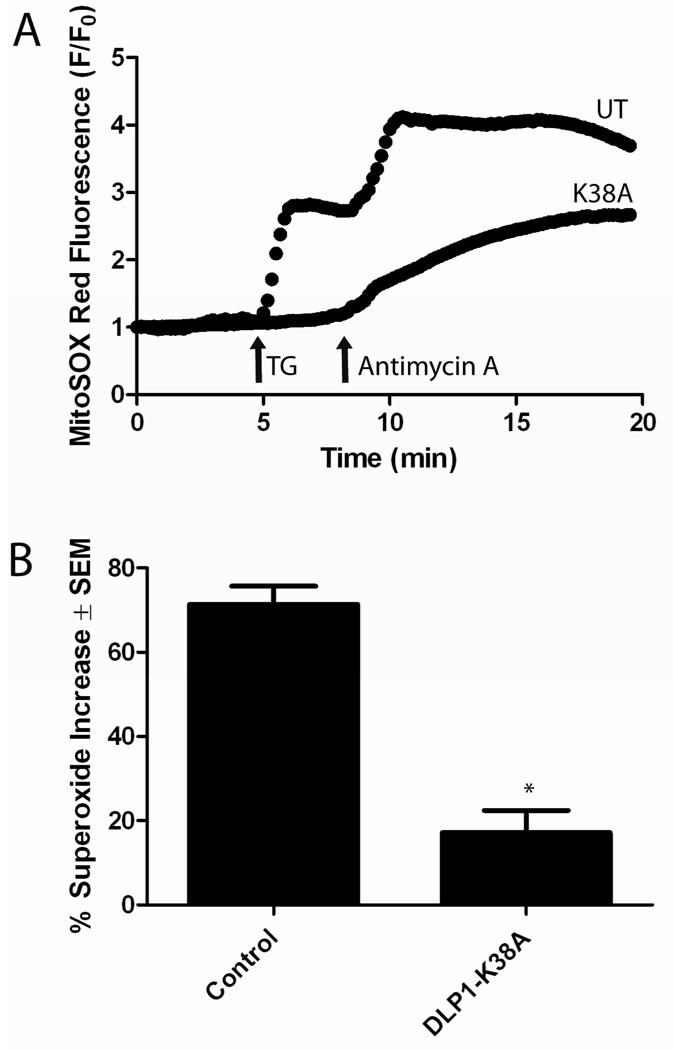
3.4. Superoxide generation with TG coincides with mitochondrial fragmentation in neonatal ventricular myocytes
An increase in mitochondrial Ca2+ can increase ROS generation by several mechanisms, including mitochondrial fission [42, 43]. Therefore, we examined the link between TG-induced cytosolic Ca2+ increase, mitochondrial fragmentation, and ROS generation. To test the effects of TG on ROS production, we performed MitoSOX Red imaging using neonatal myocytes with and without transfection with DLP1-K38A. Upon the addition of 1 µM TG to control cells, ROS production increased by approximately 70% (Fig. 4A). Addition of 10 µM antimycin A, a complex III inhibitor, induced maximum superoxide production. In contrast, overexpression of DLP1-K38A significantly attenuated the TG-induced superoxide increase, as the superoxide only increased by approximately 20% (Fig. 4A). Figure 4B shows the summarized results. The block of TG-induced superoxide increase due to DLP1-K38A overexpression suggests that DLP1 (mitochondrial fission) is necessary for TG-induced superoxide increase. These data may also indicate that Ca2+-induced mitochondrial fission occurs upstream of ROS production.
Figure 4. Inhibition of mitochondrial fission prevents Ca2+-induced superoxide increase in neonatal ventricular myocytes.
(A) Single traces of superoxide production, measured using MitoSOX Red, show that DLP1-K38A overexpression prevented TG-induced superoxide production but not that caused by 10 µM antimycin A, a mitochondrial complex III inhibitor. (B) Statistical analysis revealed that the increase in superoxide production after TG treatment was significantly decreased in cells expressing DLP1-K38A (* P<0.01, t-test, n=8).
4. DISCUSSION
4.1. Ca2+ signals regulate cardiac mitochondrial morphology
Despite the presence of mitochondrial fission proteins in the heart, there is currently very little evidence that cardiac mitochondria undergo fragmentation. In addition, elevated levels of cytosolic Ca2+ are known to induce mitochondrial fragmentation in various cell types [31]. Since cardiac myocytes depend on large cyclic changes in Ca2+, we tested whether mitochondria from these cells undergo fragmentation and if this process is regulated by Ca2+ and contributes to Ca2+-mediated mitochondrial ROS generation.
Using fluorescence imaging techniques, we observed that in neonatal ventricular myocytes, TG or high KCl induced a change from normal globular and tubular mitochondria to small, round, punctate mitochondria, indicating that mitochondria in immature myocytes can undergo fragmentation in response to Ca2+ signals. Because of the limitations in observing specific changes in adult cardiac mitochondrial morphology using fluorescence microscopy, we used electron microscopy to determine whether similar changes occur in adult mitochondria. Electron micrographs revealed that the increased cytosolic Ca2+ induced by TG also caused a reversible mitochondrial fragmentation in adult cardiac myocytes, which had smaller area, FF, and AR at 5 min after treatment. After the mitochondrial fragmentation at 5 min, however, the presence of the very large mitochondria observed at 10 min may indicate not only a recovery of normal mitochondrial morphology, but an “overshoot” of mitochondrial fusion in response to the excessive mitochondrial fission as observed previously [31]. These results are among the first to show that mature cardiac myocytes undergo fragmentation and demonstrate that this process is under the control of intracellular Ca2+ levels.
We acknowledge some limitations to these analyses of mitochondrial morphology in adult cardiac myocytes. Although our morphometry data indicate changes in mitochondrial morphology, 70 nm sections of electron micrograph do not reveal the extended structure of an individual mitochondrion. Perhaps future experiments using 3-dimensional reconstruction of confocal or EM data will better define global changes in mitochondrial morphology induced by elevated Ca2+.
TG is known to have other side effects on cellular physiology due to prolonged depletion of ER/SR Ca2+, which leads to the misfolding of proteins, activation of caspases, and cell death [44, 45]. Mitochondrial fragmentation is also associated with apoptosis and therefore it is important to consider whether our TG incubations cause pathological fragmentation of mitochondria. In our previous study, Clone 9 cells underwent rapid fragmentation of mitochondria during the first 60 minute exposure to TG without evidence of cell death. Only after 32 hours of prolonged exposure to TG, apoptosis-associated mitochondrial fragmentation was observed [31]. We found a similar reversal of mitochondrial fragmentation and no overt evidence of cell death in cardiac myocytes treated for 60 min with TG. Therefore, it is unlikely that the mitochondrial fragmentation we observed in our experiments was associated with apoptosis from prolonged exposure to TG.
4.2. Ca2+-induced mitochondrial fragmentation involves DLP1
We next tested if TG-induced mitochondrial fragmentation in cardiac myocytes requires the known mitochondrial fission process which involves translocation of cytoplasmic DLP1 to mitochondria. We observed that in adult cardiac myocytes, TG treatment caused DLP1 translocation from the cytosol to the mitochondria, an early step in mitochondrial fission. Interestingly, DLP1 remained on the mitochondria up to 30 minutes after TG treatment, but mitochondrial fragmentation reversed as early as 10 minutes after TG treatment. Previous evidence that endogenous DLP1 remains bound to mitochondria after fission events have occurred may explain this phenomenon [28]. In addition, we found that DLP1-K38A expression causes the mitochondria to form elongated tubules which, unlike in untransfected cells, did not fragment upon TG treatment. Together, these data suggest that the mechanism of Ca2+ dependent mitochondrial fragmentation in cardiac myocytes involves the known fission machinery.
What are the mechanisms by which an increase in intracellular Ca2+ leads to the activation of the mitochondrial fission machinery? There may be both mitochondrial and cytosolic pathways that activate hFis1 and DLP1. Some data suggest that mitochondrial Ca2+ uptake is necessary for mitochondrial fragmentation in different cell types [31, 46]. This agrees with our Ru360 results, which effectively blocked TG-induced mitochondrial Ca2+ uptake and fragmentation in cultured neonatal ventricular myocytes. It may be possible that hFis1, which has been shown to regulate DLP1 recruitment and assembly, may interact with inner mitochondrial proteins or membrane spanning protein complexes that are regulated by Ca2+. However, Ca2+ signaling pathways in the cytosol have been shown to regulate mitochondrial morphology via phosphorylation and dephosphorylation of DLP1 [47–52]. Future experiments will be necessary to elucidate how mitochondrial Ca2 increase activates the mitochondrial fission machinery, and if this is a different pathway than DLP1 activation.
4.3. Ca2+ induction of ROS generation involves mitochondrial fragmentation
We then tested the role of Ca2+-induced mitochondrial fragmentation and DLP1 on ROS generation, which reflects mitochondrial respiration as well as pathology. We found that TG caused an increase in intracellular Ca2+ followed by mitochondrial fragmentation and an increase in ROS. DLP1 appears to be involved in this, as our data indicate that inhibiting fission with DLP1-K38A abated a rise in ROS. This suggests that the increase in ROS is not immediately downstream of increased intracellular Ca2+, and that DLP1 and/or mitochondrial fragmentation contribute to the TG-induced ROS increase. It remains unknown whether the mitochondrial fission-mediated and electron transport chain (ETC)-mediated ROS generating mechanisms are separate and additive, or if DLP1 plays a role in modulating ETC-mediated ROS generation, as has been suggested previously [41]. On one hand, the effects of TG and ETC-mediated ROS generation could be additive, as the absolute rise in ROS after antimycin A is approximately the same in both the untreated and the DLP1-K38A cells (Fig. 4A). Interestingly, our preliminary evidence suggests that DLP1 may play a role in modulating the ROS generation from the ETC (Yoon, unpublished observations). It should also be noted that our experiments showing that the rate of antimycin A-induced ROS increase was slower in DLP1-K38A cells (Figure 4A), may also suggest this interaction. In addition, these treatments may have induced a pathologic state, in which mitochondria produce ROS associated with the mitochondrial permeability transition pore (mPTP) opening. However, as discussed above, our previous results suggest that short term TG exposure does not induce these pathologic changes. Finally, these treatments may induce a nonpathological opening of the mPTP involving superoxide flashes [53]. Future experiments with the measurements of mitochondrial membrane potential in the presence and absence of blockers for mPTP will be useful to resolve these probable mechanisms.
5. CONCLUSIONS
In conclusion, we tested the role of Ca2+ in controlling mitochondrial morphology in cardiac myocytes and provide some of the first evidence that mitochondrial fragmentation occurs in neonatal and adult cardiac myocytes. This dynamic change in mitochondrial shape is linked to the functional activity of the mitochondria, as TG increased superoxide production. In addition, these Ca2+ dependent changes in mitochondrial structure and function were mediated by the fission protein DLP1. This indicates that Ca2+ is one common factor that regulates both cardiac mitochondrial form and function in the heart.
ACKNOWLEDGMENTS
We gratefully acknowledge support from National Institutes of Health Grants DK 61991, DK 73858 and HL 33333. We thank Karen Bentley and the University of Rochester Electron Microscope core, Gisela Beutner, Donna DiManno, and Virendra Sharma who provided help during the research, and discussions with the members of Mitochondrial Research & Innovation Group.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Hom J, Sheu SS. Morphological dynamics of mitochondria--a special emphasis on cardiac muscle cells. J Mol Cell Cardiol. 2009;46:811–820. doi: 10.1016/j.yjmcc.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma VK, Ramesh V, Franzini-Armstrong C, Sheu SS. Transport of Ca2+ from sarcoplasmic reticulum to mitochondria in rat ventricular myocytes. J Bioenerg Biomembr. 2000;32:97–104. doi: 10.1023/a:1005520714221. [DOI] [PubMed] [Google Scholar]
- 3.Santel A, Frank S, Gaume B, Herrler M, Youle RJ, Fuller MT. Mitofusin-1 protein is a generally expressed mediator of mitochondrial fusion in mammalian cells. J Cell Sci. 2003;116:2763–2774. doi: 10.1242/jcs.00479. [DOI] [PubMed] [Google Scholar]
- 4.Rojo M, Legros F, Chateau D, Lombes A. Membrane topology and mitochondrial targeting of mitofusins, ubiquitous mammalian homologs of the transmembrane GTPase Fzo. J Cell Sci. 2002;115:1663–1674. doi: 10.1242/jcs.115.8.1663. [DOI] [PubMed] [Google Scholar]
- 5.Bach D, Pich S, Soriano FX, Vega N, Baumgartner B, Oriola J, Daugaard JR, Lloberas J, Camps M, Zierath JR, Rabasa-Lhoret R, Wallberg-Henriksson H, Laville M, Palacin M, Vidal H, Rivera F, Brand M, Zorzano A. Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism. A novel regulatory mechanism altered in obesity. J Biol Chem. 2003;278:17190–17197. doi: 10.1074/jbc.M212754200. [DOI] [PubMed] [Google Scholar]
- 6.Imoto M, Tachibana I, Urrutia R. Identification and functional characterization of a novel human protein highly related to the yeast dynamin-like GTPase Vps1p. J Cell Sci. 1998;111(Pt 10):1341–1349. doi: 10.1242/jcs.111.10.1341. [DOI] [PubMed] [Google Scholar]
- 7.Akepati VR, Muller EC, Otto A, Strauss HM, Portwich M, Alexander C. Characterization of OPA1 isoforms isolated from mouse tissues. J Neurochem. 2008;106:372–383. doi: 10.1111/j.1471-4159.2008.05401.x. [DOI] [PubMed] [Google Scholar]
- 8.Schaper J, Froede R, Hein S, Buck A, Hashizume H, Speiser B, Friedl A, Bleese N. Impairment of the myocardial ultrastructure and changes of the cytoskeleton in dilated cardiomyopathy. Circulation. 1991;83:504–514. doi: 10.1161/01.cir.83.2.504. [DOI] [PubMed] [Google Scholar]
- 9.Scholz D, Diener W, Schaper J. Altered nucleus/cytoplasm relationship and degenerative structural changes in human dilated cardiomyopathy. Cardioscience. 1994;5:127–138. [PubMed] [Google Scholar]
- 10.Ausma J, Thone F, Dispersyn GD, Flameng W, Vanoverschelde JL, Ramaekers FC, Borgers M. Dedifferentiated cardiomyocytes from chronic hibernating myocardium are ischemia-tolerant. Mol Cell Biochem. 1998;186:159–168. [PubMed] [Google Scholar]
- 11.Kalra DK, Zoghbi WA. Myocardial hibernation in coronary artery disease. Curr Atheroscler Rep. 2002;4:149–155. doi: 10.1007/s11883-002-0039-x. [DOI] [PubMed] [Google Scholar]
- 12.Trillo AA, Holleman IL, White JT. Presence of satellite cells in a cardiac rhabdomyoma. Histopathology. 1978;2:215–223. doi: 10.1111/j.1365-2559.1978.tb01711.x. [DOI] [PubMed] [Google Scholar]
- 13.Jones M, Ferrans VJ, Morrow AG, Roberts WC. Ultrastructure of crista supraventricularis muscle in patients with congenital heart diseases associated with right ventricular outflow tract obstruction. Circulation. 1975;51:39–67. doi: 10.1161/01.cir.51.1.39. [DOI] [PubMed] [Google Scholar]
- 14.Terman A, Dalen H, Eaton JW, Neuzil J, Brunk UT. Aging of cardiac myocytes in culture: oxidative stress, lipofuscin accumulation, and mitochondrial turnover. Ann N Y Acad Sci. 2004;1019:70–77. doi: 10.1196/annals.1297.015. [DOI] [PubMed] [Google Scholar]
- 15.Waterham HR, Koster J, van Roermund CW, Mooyer PA, Wanders RJ, Leonard JV. A lethal defect of mitochondrial and peroxisomal fission. N Engl J Med. 2007;356:1736–1741. doi: 10.1056/NEJMoa064436. [DOI] [PubMed] [Google Scholar]
- 16.Labrousse AM, Zappaterra MD, Rube DA, van der Bliek AM. C. elegans dynamin-related protein DRP-1 controls severing of the mitochondrial outer membrane. Mol Cell. 1999;4:815–826. doi: 10.1016/s1097-2765(00)80391-3. [DOI] [PubMed] [Google Scholar]
- 17.Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160:189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen H, Chomyn A, Chan DC. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem. 2005;280:26185–26192. doi: 10.1074/jbc.M503062200. [DOI] [PubMed] [Google Scholar]
- 19.Pareyson D. Differential diagnosis of Charcot-Marie-Tooth disease and related neuropathies. Neurol Sci. 2004;25:72–82. doi: 10.1007/s10072-004-0233-4. [DOI] [PubMed] [Google Scholar]
- 20.Zuchner S, Mersiyanova IV, Muglia M, Bissar-Tadmouri N, Rochelle J, Dadali EL, Zappia M, Nelis E, Patitucci A, Senderek J, Parman Y, Evgrafov O, Jonghe PD, Takahashi Y, Tsuji S, Pericak-Vance MA, Quattrone A, Battaloglu E, Polyakov AV, Timmerman V, Schroder JM, Vance JM. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat Genet. 2004;36:449–451. doi: 10.1038/ng1341. [DOI] [PubMed] [Google Scholar]
- 21.Alexander C, Votruba M, Pesch UE, Thiselton DL, Mayer S, Moore A, Rodriguez M, Kellner U, Leo-Kottler B, Auburger G, Bhattacharya SS, Wissinger B. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet. 2000;26:211–215. doi: 10.1038/79944. [DOI] [PubMed] [Google Scholar]
- 22.Delettre C, Lenaers G, Griffoin JM, Gigarel N, Lorenzo C, Belenguer P, Pelloquin L, Grosgeorge J, Turc-Carel C, Perret E, Astarie-Dequeker C, Lasquellec L, Arnaud B, Ducommun B, Kaplan J, Hamel CP. Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat Genet. 2000;26:207–210. doi: 10.1038/79936. [DOI] [PubMed] [Google Scholar]
- 23.Yoon Y, Krueger EW, Oswald BJ, McNiven MA. The mitochondrial protein hFis1 regulates mitochondrial fission in mammalian cells through an interaction with the dynamin-like protein DLP1. Mol Cell Biol. 2003;23:5409–5420. doi: 10.1128/MCB.23.15.5409-5420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu T, Fox RJ, Burwell LS, Yoon Y. Regulation of mitochondrial fission and apoptosis by the mitochondrial outer membrane protein hFis1. J Cell Sci. 2005;118:4141–4151. doi: 10.1242/jcs.02537. [DOI] [PubMed] [Google Scholar]
- 25.Ingerman E, Perkins EM, Marino M, Mears JA, McCaffery JM, Hinshaw JE, Nunnari J. Dnm1 forms spirals that are structurally tailored to fit mitochondria. J Cell Biol. 2005;170:1021–1027. doi: 10.1083/jcb.200506078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smirnova E, Shurland DL, Ryazantsev SN, van der Bliek AM. A human dynamin-related protein controls the distribution of mitochondria. J Cell Biol. 1998;143:351–358. doi: 10.1083/jcb.143.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pitts KR, Yoon Y, Krueger EW, McNiven MA. The dynamin-like protein DLP1 is essential for normal distribution and morphology of the endoplasmic reticulum and mitochondria in mammalian cells. Mol Biol Cell. 1999;10:4403–4417. doi: 10.1091/mbc.10.12.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smirnova E, Griparic L, Shurland DL, van der Bliek AM. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell. 2001;12:2245–2256. doi: 10.1091/mbc.12.8.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoon Y, Pitts KR, McNiven MA. Mammalian dynamin-like protein DLP1 tubulates membranes. Mol Biol Cell. 2001;12:2894–2905. doi: 10.1091/mbc.12.9.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hom JR, Gewandter JS, Michael L, Sheu SS, Yoon Y. Thapsigargin induces biphasic fragmentation of mitochondria through calcium-mediated mitochondrial fission and apoptosis. J Cell Physiol. 2007;212:498–508. doi: 10.1002/jcp.21051. [DOI] [PubMed] [Google Scholar]
- 32.Colecraft HM, Egamino JP, Sharma VK, Sheu SS. Signaling mechanisms underlying muscarinic receptor-mediated increase in contraction rate in cultured heart cells. J Biol Chem. 1998;273:32158–32166. doi: 10.1074/jbc.273.48.32158. [DOI] [PubMed] [Google Scholar]
- 33.Sheu SS, Sharma VK, Banerjee SP. Measurement of cytosolic free calcium concentration in isolated rat ventricular myocytes with quin 2. Circ Res. 1984;55:830–834. doi: 10.1161/01.res.55.6.830. [DOI] [PubMed] [Google Scholar]
- 34.Sharma VK, Colecraft HM, Wang DX, Levey AI, Grigorenko EV, Yeh HH, Sheu SS. Molecular and functional identification of m1 muscarinic acetylcholine receptors in rat ventricular myocytes. Circ Res. 1996;79:86–93. doi: 10.1161/01.res.79.1.86. [DOI] [PubMed] [Google Scholar]
- 35.Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci U S A. 2006;103:2653–2658. doi: 10.1073/pnas.0511154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henley JR, McNiven MA. Association of a dynamin-like protein with the Golgi apparatus in mammalian cells. J Cell Biol. 1996;133:761–775. doi: 10.1083/jcb.133.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koopman WJ, Verkaart S, Visch HJ, van der Westhuizen FH, Murphy MP, van den Heuvel LW, Smeitink JA, Willems PH. Inhibition of complex I of the electron transport chain causes O2−. -mediated mitochondrial outgrowth. Am J Physiol Cell Physiol. 2005;288:C1440–C1450. doi: 10.1152/ajpcell.00607.2004. [DOI] [PubMed] [Google Scholar]
- 38.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 39.Yoon Y, Pitts KR, Dahan S, McNiven MA. A novel dynamin-like protein associates with cytoplasmic vesicles and tubules of the endoplasmic reticulum in mammalian cells. J Cell Biol. 1998;140:779–793. doi: 10.1083/jcb.140.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lantum HB, Baggs RB, Krenitsky DM, Anders MW. Nephrotoxicity of chlorofluoroacetic acid in rats. Toxicol Sci. 2002;70:261–268. doi: 10.1093/toxsci/70.2.261. [DOI] [PubMed] [Google Scholar]
- 41.Treiman M, Caspersen C, Christensen SB. A tool coming of age: thapsigargin as an inhibitor of sarco-endoplasmic reticulum Ca(2+)-ATPases. Trends Pharmacol Sci. 1998;1:131–135. doi: 10.1016/s0165-6147(98)01184-5. [DOI] [PubMed] [Google Scholar]
- 42.Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 43.Yu T, Sheu SS, Robotham JL, Yoon Y. Mitochondrial fission mediates high glucose-induced cell death through elevated production of reactive oxygen species. Cardiovasc Res. 2008;79:341–351. doi: 10.1093/cvr/cvn104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaneko Y, Tsukamoto A. Thapsigargin-induced persistent intracellular calcium pool depletion and apoptosis in human hepatoma cells. Cancer Lett. 1994;79:147–155. doi: 10.1016/0304-3835(94)90253-4. [DOI] [PubMed] [Google Scholar]
- 45.Jiang S, Chow SC, Nicotera P, Orrenius S. Intracellular Ca2+ signals activate apoptosis in thymocytes: studies using the Ca(2+)-ATPase inhibitor thapsigargin. Exp Cell Res. 1994;212:84–92. doi: 10.1006/excr.1994.1121. [DOI] [PubMed] [Google Scholar]
- 46.Breckenridge DG, Stojanovic M, Marcellus RC, Shore GC. Caspase cleavage product of BAP31 induces mitochondrial fission through endoplasmic reticulum calcium signals, enhancing cytochrome c release to the cytosol. J Cell Biol. 2003;160:1115–1127. doi: 10.1083/jcb.200212059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Han XJ, Lu YF, Li SA, Tomizawa K, Takei K, Matsushita M, Matsui H. Involvement of calcineurin in glutamate-induced mitochondrial dynamics in neurons. Neurosci Res. 2008;60:114–119. doi: 10.1016/j.neures.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 48.Harder Z, Zunino R, McBride H. Sumo1 conjugates mitochondrial substrates and participates in mitochondrial fission. Curr Biol. 2004;14:340–345. doi: 10.1016/j.cub.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 49.Nakamura N, Kimura Y, Tokuda M, Honda S, Hirose S. MARCH-V is a novel mitofusin 2- and Drp1-binding protein able to change mitochondrial morphology. EMBO Rep. 2006;7:1019–1022. doi: 10.1038/sj.embor.7400790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taguchi N, Ishihara N, Jofuku A, Oka T, Mihara K. Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J Biol Chem. 2007;282:11521–11529. doi: 10.1074/jbc.M607279200. [DOI] [PubMed] [Google Scholar]
- 51.Chang CR, Blackstone C. Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J Biol Chem. 2007;282:21583–21587. doi: 10.1074/jbc.C700083200. [DOI] [PubMed] [Google Scholar]
- 52.Cribbs JT, Strack S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep. 2007;8:939–944. doi: 10.1038/sj.embor.7401062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang W, Fang H, Groom L, Cheng A, Zhang W, Liu J, Wang X, Li K, Han P, Zheng M, Yin J, Mattson MP, Kao JP, Lakatta EG, Sheu SS, Ouyang K, Chen J, Dirksen RT, Cheng H. Superoxide flashes in single mitochondria. Cell. 2008;134:279–290. doi: 10.1016/j.cell.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]