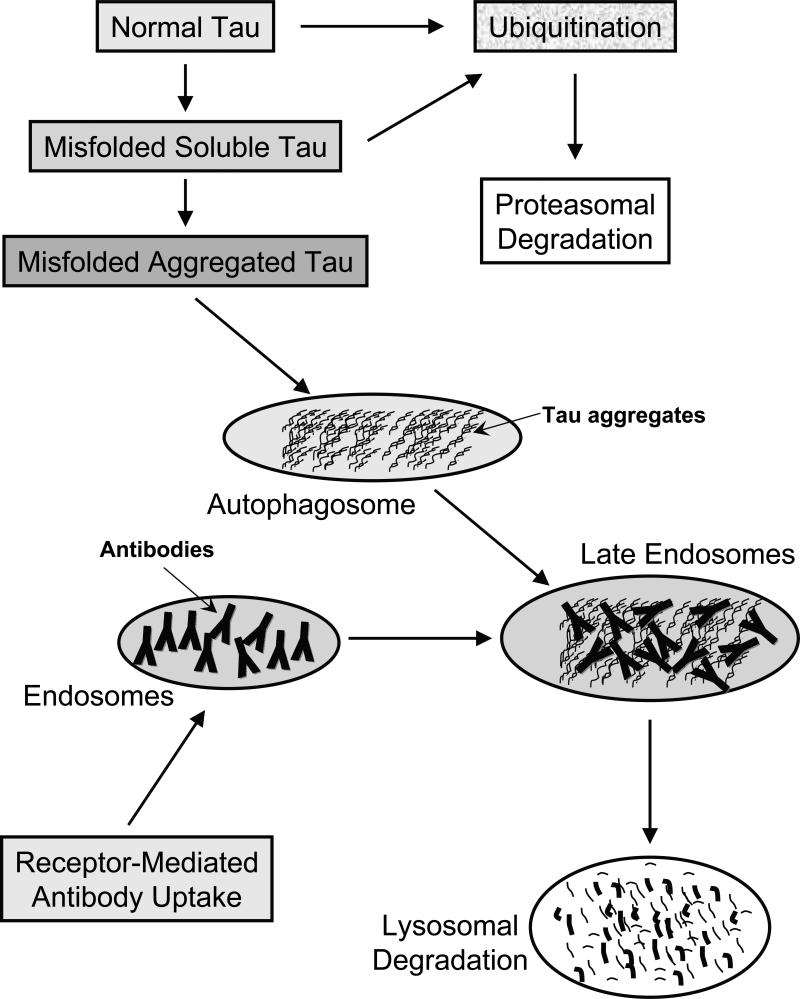
Figure 1. Clearance of normal and pathological tau within neurons.
Normal tau is likely to be marked by ubiquitination for subsequent proteasomal degradation. Misfolded soluble tau should be cleared by the same route whereas once it aggregates, those assemblies are presumably too large for the proteosome and could be encapsulated within the autophagosome.
Receptor-mediated antibody uptake will place those in endosomes that can then fuse with autophagosomes in late endosomes, in which the antibodies may bind to the tau aggregates. At that stage and/or when the endosomes fuse with lysosomes, these antibodies may promote disassembly of the aggregates that should facilitate their cleavage by lysosomal enzymes.