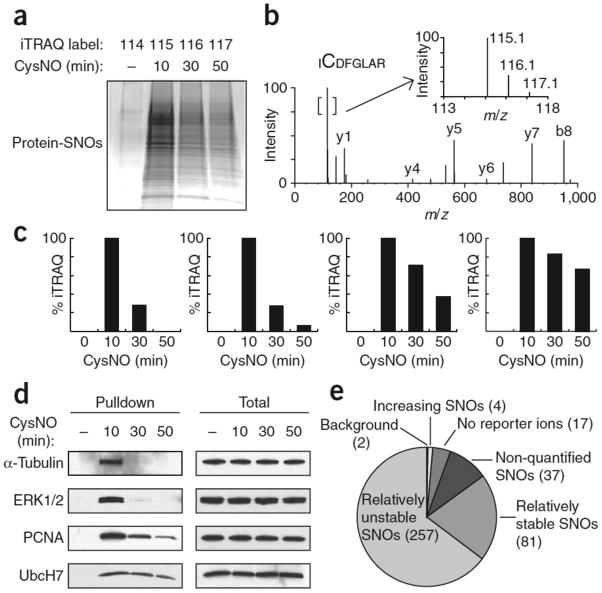
Figure 2. iTRAQ-coupled SNO-RAC demonstrates proteome-wide (global) denitrosylation.
HEK293 cells were treated ±0.5 mM CysNO for 10, 30 or 50 min and subjected to SNO-RAC. (a) Samples were analyzed by SDS-PAGE with Coomassie staining or (b–e) subjected to on-resin trypsinization and labeling with iTRAQ reagents, yielding peptides with reporter ions at 114, 115, 116 and 117 amu for untreated samples (0 min) and 10, 30 and 50 min post-CysNO treatment, respectively. (b) A representative MS/MS spectrum containing SNO-Cys166 in Erk2. The inset shows an expanded view of the reporter ion region. (c) SNO sites corresponding to α-tubulin Cys347 and Erk2 Cys166, with a 115/116 ratio > 1.5 (‘unstable’), and PCNA Cys162 and UbcH7 Cys86, which possess a 115/116 ratio between 0.6 and 1.5 (‘stable’). (d) Verification of the iTRAQ data in c by western blotting for intact SNO proteins. (e) A global kinetic analysis of SNO stability via iTRAQ ratios identified 398 unique peptides, 396 of which contained a cysteine residue. The vast majority of peptides were relatively “unstable” (257 exhibited 115/116 > 1.5). Additional SNO peptides included: 81 with 115/116 between 0.6 and 1.5 (relatively ‘stable’); 2 possessed barely appreciable reporter ion at 114 (115/114 ratio < 2.5) (‘background’); 17 were without detectable reporter ion; 37 possessed reporter ion at 115 amu only. Four SNO sites appeared to increase over time (115/116 < 0.6). Full-length scans are available in Supplementary Figure 7.