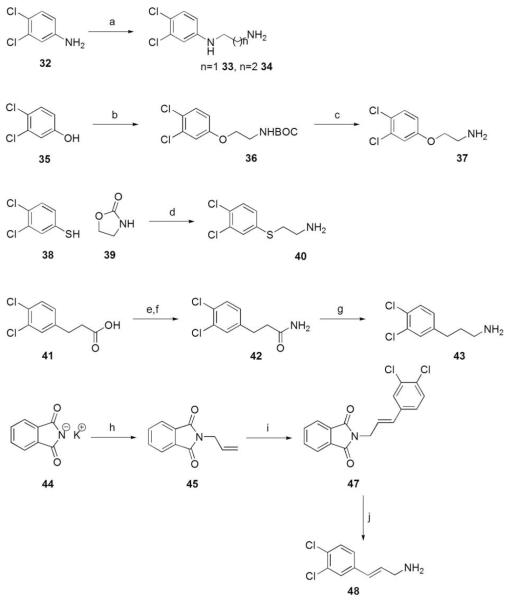
Scheme 4.
Reagents and conditions: (a) bromoethylamine hydrobromide or bromopropylamine HBr, toluene, reflux; (b) N-Boc-aminoethanol, PPh3, DIAD, THF, 0 °C to rt; (c) TFA, THF, rt; (d) 130 °C; (e) oxalyl choride, THF, DMF, rt; (f) Et2O, NH3 in 1,4-dioxane, rt; (g) LiAlH4, THF, 0 °C to rt; (h) allyl bromide, DMF, 50 °C; (i) 3,4-dichloroiodobenzene (46), Pd(OAc)2,Et3N, MeCN, 100 °C; (j) NH2NH2·H2O, EtOH, reflux.