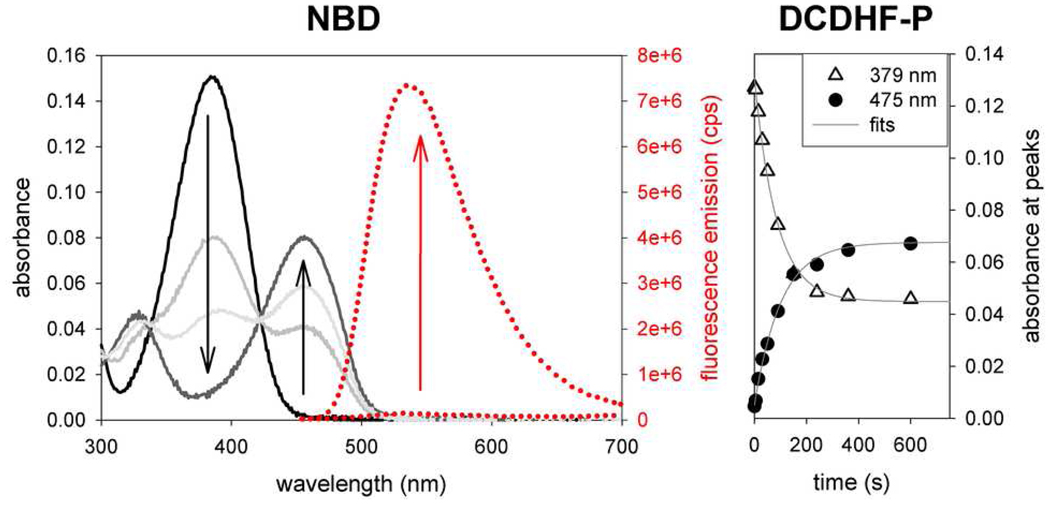
Figure 1.
(left) Spectra of NBD-azide photoactivation. (See the SI for spectra of the other fluorogens.) Because the azide cannot participate as a donor in a charge transfer, the fluorogen exhibits blue-shifted absorption (λabs = 384 nm) with respect to its amine-donor sister (λabs = 456 nm). Upon irradiation with UV light, the azido fluorogen (solid black curve) converts to the amino fluorophore (solid grey curves). Fluorescence (dotted red lines) excited at 440 nm increases significantly after photoconversion (right) Photoactivation of DCDHF-P-azide using a 385-nm flashlight (1.1 mW cm−2). The short-wavelength azido absorption peak (triangles) disappears with time and the long-wavelength absorption peak (circles) corresponding to the fluorescent amino fluorophore grows in. The time constant (τP = 85 s) from the exponential fit of the disappearance of the azido fluorogen is used to calculate the photoconversion quantum yield (ΦP) in Table 1.