Fig. 6.
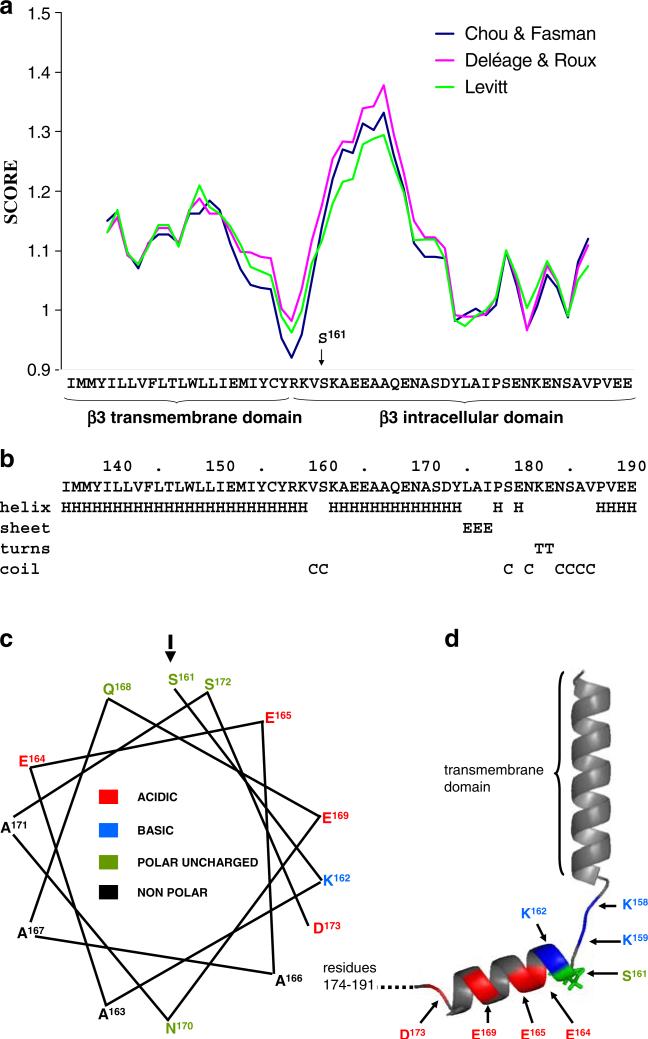
Secondary structure prediction of β3 transmembrane and intracellular domains. a The propensity of the putative transmembrane and intracellular domains of human β3 (residues I136 to E191 of the mature protein) to fold into an α-helix were predicted using a window size of nine residues and the algorithms of Deléage and Roux [6], Chou and Fasman [4], and Levitt [17]. b Secondary structure prediction using the method of Garnier et al. [7]. Residues K163 to D174 are strongly predicted to fold into an α-helix comprising hydrophobic residues with changed residues at every third or fourth position. c Helical wheel projection of the putative intracellular domain helix (amino acids 161–173) showing its amphipathic nature and the position of the potentially phosphorylated S161. d Structural model of the transmembrane domain and the intracellular domain of the β3 emphasizing short disordered sequence separating helices. The model was built using pyMol