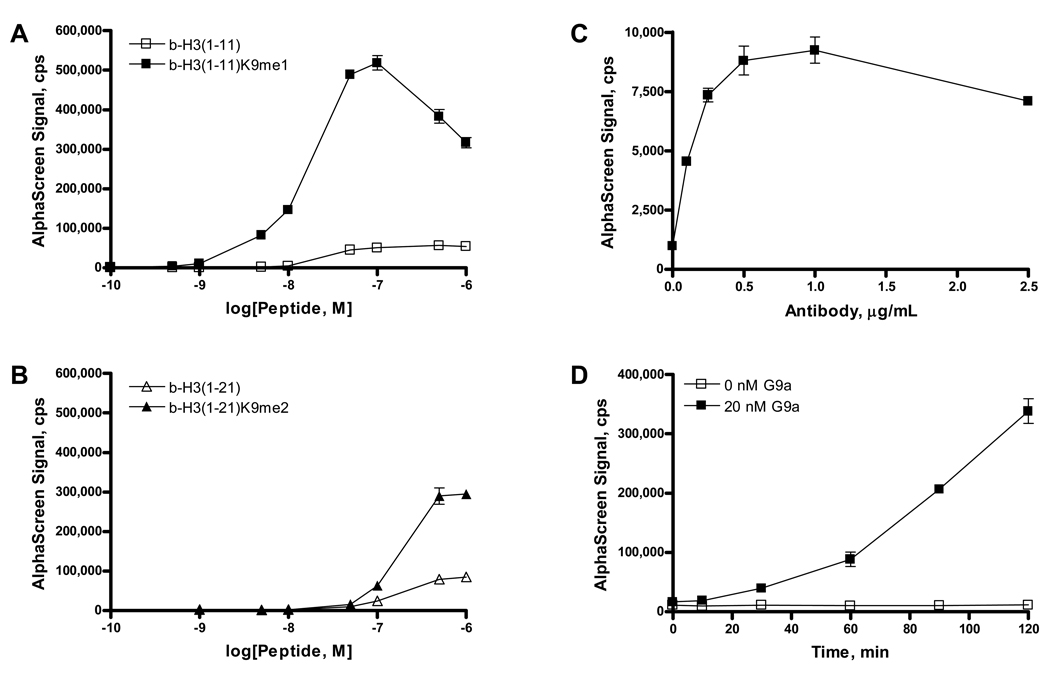
Figure 1.
Specificity of binding of a polyclonal antibody to human histone H3 methylated at Lys9 was determined with biotinylated peptides of differing methylation states. A, Antibody binding to monomethylated Lys9 was observed as increasing AlphaScreen signal at concentrations up to 100 nM b-H3(1–11)K9me1. B, Binding of antibody to dimethylated Lys9 was also observed, and AlphaScreen signal increased with increasing peptide concentration up to 500 nM b-H3(1–21)me2. Corresponding unmethylated peptides displayed low affinity for antibody binding. C, Optimization of antibody concentration was determined in incubations with 1.0 nM b-H3K9me1 peptide. D, Reaction progress of b-H3(1–11) methylation by 20 nM G9a was measured in 384-well plate format in incubations containing 20 µM SAM and 500 nM peptide substrate. The mean and standard deviation of triplicate measurements are shown.