Table 2.
Intermolecular Hydroamination of Alkenes with 1 (0.4 M) Catalyzed by a Mixture of (L)AuCl (5 mol %) (L = 2a, 2b) and AgSbF6 (5 mol %) in Dioxane at 100 °C.
| entry | alkene | alkene amt | product | L | time (h) | yield (%)a |
|---|---|---|---|---|---|---|
|
|
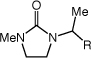
|
|||||
| 1 | R = H | 60 psi b | 3 | 2b | 24 | 99 |
| 2 | R = Me | 120 psi | 2b | 24 | 98 | |
| 3 | R = Et | 120 psi | 2b | 24 | 96 | |
| 4 | R = (CH2)5CH3 | 10 equiv | 2b | 24 | 96 | |
| 5 | R = (CH2)3OH | 10 equiv | 2b | 24 | 98 | |
| 6 | R = (CH2)3OBn | 10 equiv | 2b | 40 | 95 | |
| 7 | R = (CH2)2CO2H | 10 equiv | 2a | 40 | 98 | |
| 8 | R = (CH2)4CO2Et | 10 equiv | 2a | 40 | 90 | |
| 9 | R = Ph | 15 equiv | 2b | 36 | 75 | |
| 10 |
|
120 psic |
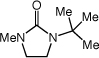
|
2a | 48 | 72 |
| 11 |
|
20 equivc |
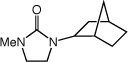
|
2b | 70 | 86d |
Yield of isolated, regiochemically pure material of >95% chemical purity.
Reaction temperature = 60 °C.
Catalyst loading = 10 mol %.
Single diastereomer formed, relative configuration not determined.
