Table 3.
Enantioselective Intermolecular Hydroamination of 1-Alkenes (60 equiv) with Imidazolidin-2-ones Catalyzed by a Mixture of [(S)-4](AuCl)2 (2.5 mol %) and AgOTf (5 mol %) in m-Xylene at 100 °C for 48 h.
| entry | nucleophile | alkene | product | yield (%)a | ee (%)b |
|---|---|---|---|---|---|
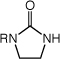
|
|
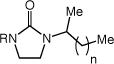
|
|||
| 1 | R = Me (1) | n = 5 | 86 | 76 | |
| 2 | R = Ph | n = 5 | 80 | 71 | |
| 3 | R = 4-C6H4F | n = 5 | 81 | 74 | |
| 4 | R = t-Bu | n = 5 | 89 | 78 | |
| 5 | R = Me (1) | n = 7 | 83 | 74 | |
| 6 | R = Me (1) | n = 9 | 76 | 75 | |
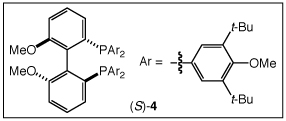
|
|||||
Yield of isolated, regiochemically pure material of >95% chemical purity.
Enantiopurity determined by HPLC analysis employing chiral stationary phase.
