Figure 1. Assay of Patient Serum for anti-Insulin Receptor Antibodies.
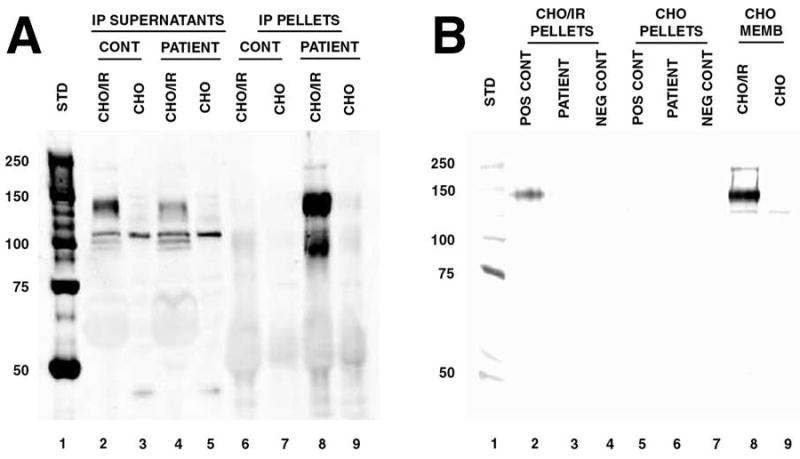
Serum was assayed for anti-insulin receptor antibodies using recombinant human insulin receptor in an immunoprecipitation assay (2). Detergent-solubilized membranes of CHO/IR cells expressing human insulin receptor (IR) were the source of insulin receptor used. The CHO/IR cells and parent CHO cells, used as controls, were obtained from Dr. Jeffrey Pessin, University of Iowa College of Medicine (7). Parallel control immunoprecipitations with pooled healthy donor serum (Irvine Scientific, Santa Ana, California) were performed in all cases. Standard protocols for immunoprecipitation and western blotting were employed (8,9). Serum samples were incubated with CHO/IR or CHO membrane lysates. Patient or control serum (50 μl) was added to the solubilized membranes and incubated at 4°C overnight, followed by addition of protein G sepharose for 1 hour at 4°C. Antibody complexes were collected by centrifugation. Precipitated antibody complexes (pellets) and residual soluble proteins (IP supernatants) were analyzed by western blotting with rabbit anti-insulin receptor α (N-20: sc-710 pAb, Santa Cruz Biotechnology, Inc.). Panel A: Assay of patient serum obtained during the presenting hospitalization. 1, molecular weight standards; 2 & 3, supernatants of CHO/IR and CHO membranes after precipitation with pooled human serum; 4 & 5, supernatants of CHO/IR and CHO membranes after precipitation with patient serum; 6 & 7, pelleted antibody complexes from solubilized CHO/IR and CHO membranes using pooled human serum; 8 & 9, pelleted antibody complexes from solubilized CHO/IR and CHO membranes using patient serum. An intense band corresponding to insulin receptor α-subunit was present in antibody precipitates formed with patient serum and solubilized CHO/IR membranes (lane 8), but not in precipitates with control CHO membranes (lane 9) or precipitates with pooled human serum (lanes 6 & 7). Depletion of insulin receptor from the CHO/IR supernatants by antibodies in the patient’s serum, but not pooled human serum from healthy donors, is also evident (lanes 2 & 4). Panel B: Assay of patient serum obtained two years after presentation. 1, molecular weight standards; 2, 3 & 4, pelleted antibody complexes from solubilized CHO/IR membranes using positive control serum (patient serum from his initial presentation), patient serum, and pooled human sera; 5, 6 & 7, pelleted antibody complexes from solubilized CHO membranes using positive control serum, patient serum, and pooled human sera; 8 & 9, CHO/IR and CHO membranes loaded directly onto the gel without immunoprecipitation to confirm insulin receptor detection.
