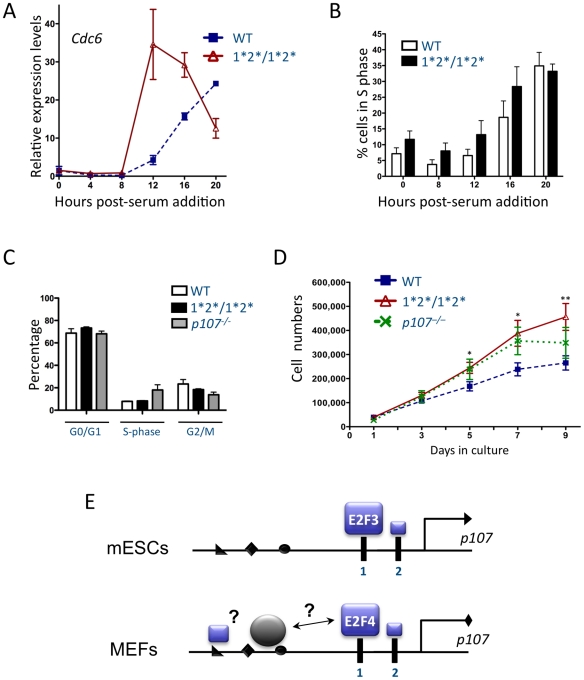
Figure 6. Altered p107 expression affects cellular proliferation.
(A,B) Immortalized wild-type and p107E2F-1*2*/1*2* MEFs were synchronized in G0 through at least three days of serum starvation. DMEM supplemented with 20% BGS was used to stimulate cell-cycle entry. Extracts were collected at the number of hours indicated post-serum stimulation. (A) RT-qPCR analysis of Cdc6 mRNA in wild-type and p107E2F-1*2*/1*2* MEFs. (n = 3) (B) Percentage of cells in S-phase, as determined by BrdU/PI staining, at the indicated time points. (n≥4) (C) Cell-cycle profiles of asynchronous primary wild-type, p107E2F-1*2*/1*2*, and p107−/− MEFs. Percentages of cells in each phase were determined by BrdU/PI staining. (n≥2) (D) Cellular proliferation of primary wild-type, p107E2F-1*2*/1*2*, and p107−/− MEFs. Equal numbers of cells were plated at day 0. Cells were then counted every other day from day 1 to day 9 post-plating. For statistical analysis, p107E2F-1*2*/1*2* cells were compared to wild-type cells at each time point. (n≥13) (E) Model for the context-dependent regulation of p107 transcription by E2F family members. In cycling mESCs, activating members of the E2F family such as E2F3 bind to the p107 promoter mostly through the distal consensus E2F binding site (site 1). In quiescent MEFs, binding of the E2F4 repressor is also largely dependent on the presence of the distal consensus site. However, E2F4 may also be recruited to the p107 promoter through interactions with other transcription factors and/or by binding to other DNA sequences. The size of the E2F boxes indicates the relative binding activity.