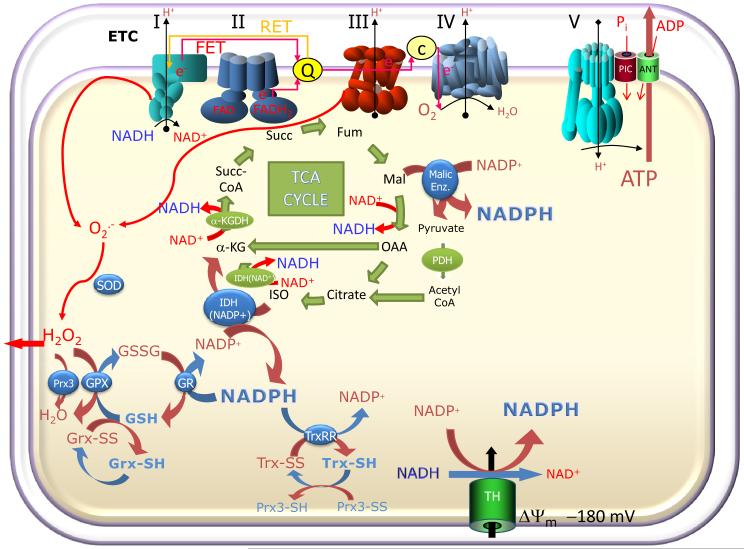
Figure 2. Moiety-conserved redox cycles linking metabolic and antioxidant pathways in mitochondria.
O2.− produced by the mitochondrial electron transport chain through reverse- or forward-electron transport (RET or FET) is dismutated to H2O2 by superoxide dismutase (SOD). The reduced redox environment is controlled by several systems, including the large capacity glutathione system (GSH, GSSG), the glutaredoxin (Grx) system, and the thioredoxin (Trx) system, responsible for the reduction of selected protein targets such as peroxiredoxin (Prx) and ribonucleotide reductase. GSH and Trx are both essential for the detoxification of H2O2 via glutathione peroxidase (GPX) and Prx enzymes, respectively. Mitochondrial catalase may also contribute to H2O2 scavenging (not shown). Different isoforms of these enzymes are present in the cytosol and in the mitochondrial matrix; however, in both compartments, their redox state depends on maintaining the negative reduction potential of NADPH (> −350 mV). The NADPH/NADP+ redox couple is, in turn, kept in the reduced state through the metabolic pathways of the cell through a close relationship between oxidative metabolism, electron transport, and antioxidants. In the matrix, three major enzymes involved in NADPH regeneration are the NAD(P) transhydrogenase (TH; which catalyzes the transfer of electrons between NADH and NADP+ at the expense of the protonmotive force), the NADP+-dependent isocitrate dehydrogenase (IDH-NADP+) and Malic enzyme; the latter two are dependent on TCA cycle intermediates. Hence, any change in oxidative phosphorylation could also affect the antioxidant pathways.