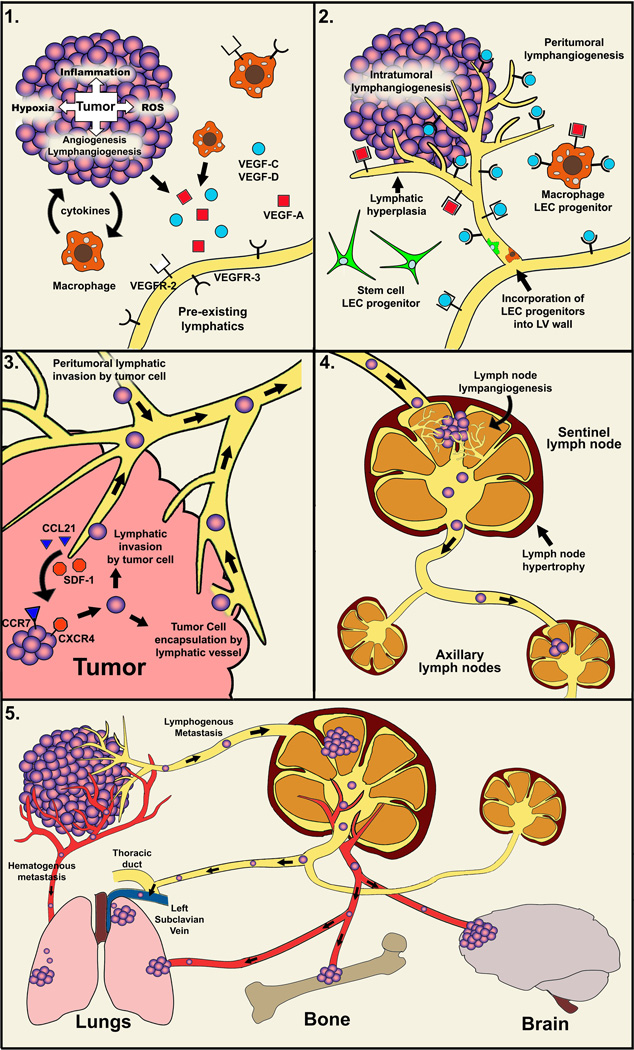
Figure 2. Mechanisms of lymphatic metastasis.
Panel 1: Inflammation, hypoxia and reactive oxygen species (ROS) up-regulate expression of pro-lymphangiogenic factors VEGF-C, VEGF-D and VEGF-A produced by both tumor neoplastic and tumor-associated stromal cells (e.g. macrophages and fibroblasts). Panel 2: New lymphatic vessels are formed mainly in the peritumoral region but in some cases also intratumorally. The formation of new lymphatic vessels involves activation of VEGFR-3 by VEGF-C/-D and VEGFR-2 by VEGF-A as well as mature forms of VEGF-C/-D. Following, LEC proliferate, migrate and assemble into new vessels. The process may include incorporation of tumor-recruited circulating LEC progenitor cells. Panel 3: Tumor cells might actively invade intratumoral or peritumoral lymphatic vessels, or might be passively encapsulated by lymphatic vessels. Both types of interactions promote LVI leading to lymphatic metastasis to the sentinel lymph node. The active metastasis includes chemotactic recruitment of tumor cells expressing CCR7 or CXCR4 receptors by corresponding chemokines (CCL21 and SDF-1) expressed by the local lymphatic system. Panel 4: Unknown tumor-secreted factors that may include, but not limited to VEGF-A and VEGF-C, elicit profound morphological changes in the sentinel LN culminating in lymph node lymphangiogenesis (LNL) and lymphatic hypertrophy. Dramatically increased lymph flow in and out of the sLN promotes both lymphatic metastasis and further dissemination to distant organs. Panel 5: The pathway to distant organs may occur through both blood and lymphatic vasculature. Following dissemination through lymphatic vessels to the sentinel LN, tumor cells may enter the blood circulation through nodal blood vessels, or proceed with the lymphatic flow and enter the venous circulation via the thoracic duct, both pathways leading to distant organ metastasis.