Abstract
Objective
To identify the predictors of suicidal events and attempts in depressed adolescent suicide attempters treated in an open treatment trial.
Method
Adolescents who had made a recent suicide attempt and had unipolar depression (n=124) were either randomized (n=22) or given a choice (n=102) among three conditions. Two participants withdrew prior to treatment assignment. The remaining 124 youth received either: a specialized psychotherapy for suicide attempting adolescents (n=17), a medication algorithm (n=14), or the combination (n=93). The participants were followed up 6 months after intake with respect to rate, timing, and predictors of a suicidal event (attempt or acute suicidal ideation necessitating emergency referral).
Results
The morbid risks of suicidal events and attempts upon 6-month follow-up were 0.19 and 0.12, respectively, with a median time to event of 44 days. Higher self-rated depression, suicidal ideation, family income, greater number of previous suicide attempts, lower maximum lethality of previous attempt, history of sexual abuse, and lower family cohesion predicted the occurrence, and earlier time to event, with similar findings for the outcome of attempts. A slower decline in suicidal ideation was associated with the occurrence of a suicidal event.
Conclusions
In this open trial, the 6-month morbid risks for suicidal events and for re-attempts were lower than in other comparable samples, suggesting that this intervention should be studied further. Important treatment targets include suicidal ideation, family cohesion, and sequelae of previous abuse. Because 40% of events occurred with 4 weeks of intake, an emphasis on safety planning and increased therapeutic contact early in treatment may be warranted.
Keywords: suicide attempt, adolescents, depression, pharmacotherapy, psychotherapy
Introduction
Suicidal behavior in adolescents is a major public health problem because of its frequency, likelihood for recurrence, health care costs, and increased risk for completed suicide.1 Despite progress in the identification of risk factors for attempted and completed suicide, there are no interventions that have been shown to reliably decrease the risk of re-attempt in adolescents who make an initial suicide attempt.1 The development of interventions to decrease the risk of re-attempt in high-risk patient populations has been identified as a national imperative.2
The development of a treatment for adolescent suicide attempters presents several challenges. First, adolescent suicide attempters often present with multiple problems, such as depression and other comorbid psychiatric conditions, health risk behaviors, and family discord, but there is no extant empirical method for determining which domains are the most salient targets for intervention.1;3;4 Second, it is difficult in the context of a clinical trial to demonstrate the prevention of suicide re-attempts, except in large samples that are enriched with high-risk individuals. Third, investigators have been reluctant to conduct any type of experimental research with suicidal individuals because of concern about the risk for completed suicide, yet progress in the clinical management of suicidal individuals is impossible without empanelling such patients into clinical trials.2;5
In light of these concerns, the NIMH and five academic medical centers, under the auspices of the Research Units on Pediatric Psychopharmacology (RUPP) developed the Treatment of Adolescent Suicide Attempters (TASA) study. The TASA group developed a combined psychosocial and pharmacological treatment protocol and tested it in a six-month trial in 124 depressed adolescent suicide attempters. Adolescents with both depression and a recent suicide attempt were studied because depressed suicide attempters are at especially high risk for a recurrent attempt.6–8 The goals of the trial were to establish the feasibility and acceptability of the intervention, and to identify factors that predict or mediate the recurrence of suicidal behavior, in order to further refine this intervention for use in a randomized clinical trial.
Herein, we describe, in a sample of depressed adolescent suicide attempters, the risk and time to occurrence of a suicidal event, and the baseline predictors thereof. Suicidal events include suicide attempts, as well as high levels of suicidal ideation that necessitate an emergency evaluation or a change in treatment plan.5 The identification of factors associated with recurrent suicidal behavior can aid in the selection of samples at high enough risk to be able to detect an intervention effect, and also can help to identify and prioritize salient treatment targets for the prevention of further suicidal episodes.
Predictors of recurrent suicidal behavior in suicide attempters, while well studied in clinical and epidemiological samples, have not been examined in depressed adolescents who have been systematically assessed and treated. Based on the literature, we predict that the risk of recurrent suicidal behavior and suicidal events will be increased in those with greater number of previous attempts, higher suicidal intent and lethality of the index attempt, higher intake suicidal ideation, presence and severity of a mood and anxiety disorders, comorbid disruptive disorder or substance abuse, and higher hopelessness, impulsivity, emotional lability, impulsive aggression, and family adversity.1;3;4;6–8
We further hypothesize that among putative treatment targets (e.g., suicidal ideation, self-reported depression), those with recurrent suicidal events and behavior will show less change over time than those who emerged from treatment without experiencing another suicidal event.
Method
Sample
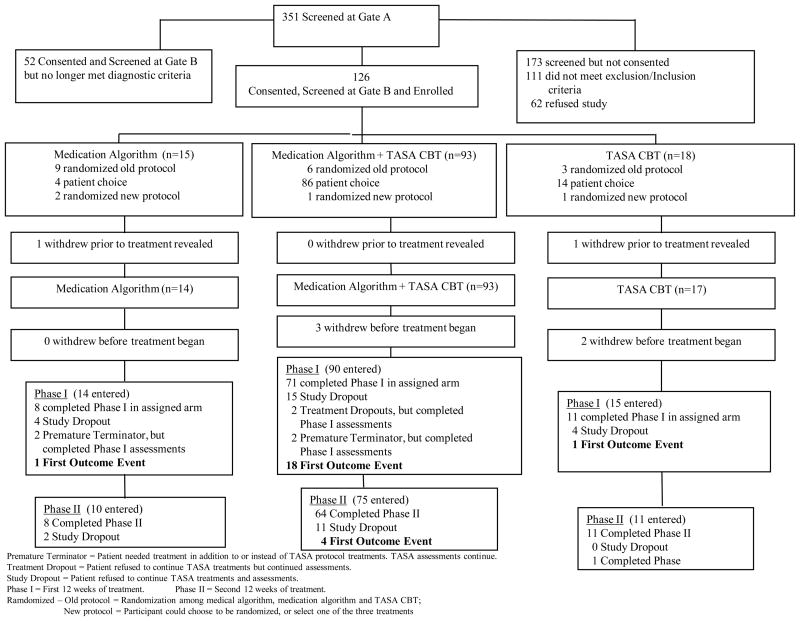
Participants were adolescents aged 12–18 years who had made a suicide attempt within 90 days of intake. A suicide attempt was defined as a “self-destructive act with explicit or inferred intent to die.”9 Participants were required to have a major unipolar mood disorder (either major depression [n=106], dysthymic disorder [n=1], depression not otherwise specified [n=4], or both major depression and dysthymia [n=13]) and had at least moderate symptoms of depression (Child Depression Rating Scale-Revised [CDRS-R] ≥ 36). Participants were required to be living with a parent or guardian who could participate in treatment, and could not have substance dependence, bipolar disorder, psychosis, or a developmental disorder. A consort chart shows the flow into the study (Figure 1).
Figure 1. Consort Chart.
Premature terminator = Patient needed treatment in addition to or instead of TASA protocol treatments. TASA assessments continue.
Treatment dropout = Patient refused to continue TASA treatments but continued assessments.
Study dropout = Patient refused to continue TASA treatments and assessments.
Phase I = First 12 weeks of treatment.
Phase II = Second 12 weeks of treatment.
Randomized Old protocol = Randomization among medical algorithm, medication algorithm and TASA CBT.
New protocol = Participant could choose to be randomized, or select one of the three treatments.
CBT = Cognitive Behavior Therapy
TASA = Treatment of Adolescent Suicide Attempters
Design (See Figure 1)
This study was started as a 3-arm randomized trial. The initial 18 participants were randomized into one of three conditions: psychotherapy, medication management, or the combination. Due to difficulties in recruitment, the design was then modified to allow participants to either be randomized (n=4) or to choose their preferred treatment (n=104). A total of 126 participants initially consented, but 2 withdrew their consent, and 5 withdrew prior to the initiation of treatment, leaving 124 participants with a baseline assessment and 119 who initiated treatment.
Treatment
Treatment consisted of a 6-month intervention, either a medication algorithm derived from the Texas Medication Algorithm,10 psychotherapy, or the combination. The psychotherapeutic treatment took as its point of departure a cognitive behavioral treatment for adult suicide attempters,11 but was extensively modified to fit the developmental and clinical needs of depressed, suicidal adolescents. This modification drew heavily from manuals developed for the Treatment of Adolescent Depression Study (TADS),12;13 Treatment of SSRI Resistant Depression in Adolescents (TORDIA),14;15 and Dialectic Behavior Therapy.16 The medication algorithm, and psychotherapy are described in greater detail in companion publications.17;18
Primary Outcome
The primary outcome for this study was a suicidal event, assessed using the Suicide Severity Rating Scale (SSRS). A suicide event, defined according to the Columbia Classification Algorithm of Suicide Assessment, and classified by a blinded panel of experts, consisted of one of the following: completed suicide, attempted suicide, preparatory acts towards imminent, suicidal behavior, or suicidal ideation. A suicide attempt was defined as an act of potentially self-injurious behavior with explicit or inferred intent to die.9 Suicidal events has been used as an endpoint in other treatment studies of suicidal behavior.5;19 Previous cross-sectional and longitudinal comparisons have shown that ideators with high intent who seek emergency referral are similar to bona fide attempters on a broad range of clinical characteristics, and often make attempts on subsequent follow-up.8;20 The impact of these treatments on depression outcomes is the focus of a related paper.21
Human Subjects Protection
This protocol was approved by all sites’ Institutional Review Boards and informed consent/assent was obtained prior to entry into the study. Study recruitment and adverse events were reported on a quarterly basis to a Data Management Safety Board (DSMB) constituted by the NIMH. In order to protect these high risk participants in this study, we took the following steps: (1) no-shows were vigorously pursued; (2) as per the study manual, each participant had a safety plan developed that including internal and external coping strategies to be implemented should the participant experience suicidal urges; (3) 24-hour clinical back-up at each site was provided; and (4) participants whose clinical status indicated that they needed a different treatment than that which TASA was providing (e.g., participant was bipolar or was psychotic) were removed and a referral was facilitated. The occurrence of a suicidal event per se was not a sufficient reason for removal. However, any participant who experienced a suicidal event was required to be evaluated by a designated ombudsperson who was independent of the study team. The role of the ombudsperson was to provide independent, clinical evaluation about the appropriateness of a participant’s continuing participation in the trial and in their assigned treatment arm following a suicidal event. The ombudsperson’s decision was binding.
Assessment
Participants were assessed with regard to suicidality at weeks 6, 12, 18, and 24 after intake. The majority (n=96, 77.4%) were assessed at week 12, with 87 assessed at week 18, and 83 at week 24. Median time of maximum assessment was 85 days (Inter-quartile Range=61.5 days). Those followed up for more than median duration, compared to those followed less than the median, had higher income (t=3.19, df=114, p<.002), but were similar with respect to other baseline predictors of recurrent suicidal events found in this sample. Baseline characteristics assessed include characteristics of past and index suicidal behavior (intent, lethality, number of previous attempts, age of first attempt), and worst and current level of suicidal ideation, assessed by the Columbia Suicide History Form,22 Beck Suicide Intent Scale23 and the Scale for Suicidal Ideation (SSI),24 respectively. Participants were assessed diagnostically using the School Aged Children Schedule for Affective Disorders and Schizophrenia, Present and Lifetime Version (K-SADS-PL).25 Characteristics of depression assessed include age of onset, duration, number of previous episodes, and interview-rated and self-reported severity of depression, using the Children’s Depression Rating Scale-Revised (CDRS-R)26 and the Beck Depression Inventory (BDI),27 respectively. Hopelessness was assessed using the self-reported Beck Hopelessness Scale,28 anxiety by the Multidimensional Anxiety Scale (MASC),29 and tendency to aggression and hostility by the Aggression Questionnaire.30 Emotional lability and impulsivity were assessed using the Emotionality, Activity, Sociability, and Impulsivity Survey (EASI),31 and an interview-rating of the number of symptoms for Borderline Personality Disorder. History of maltreatment was rated using the Childhood Experiences Questionnaire,32 and family climate of adaptability and cohesion were rated using the Family Adaptability and Cohesion Evaluation Scales (FACES-II).33
Data Analysis
Analyses were intent to treat, with last observation carried forward. We focus on suicidal events as an outcome, and report results specifically for attempts only when they diverge from the findings for suicidal events. The baseline characteristics of those who did or did not experience a suicidal event within 6 months of intake were compared to those who did not using standard univariate parametric and non-parametric statistics. A series of logistic regressions by domain were conducted to identify those baseline characteristics most closely associated with suicidal events. Those variables that emerged from these logistic regressions were then tested one by one with a logistic regression that controlled for age, race, sex, site, parental education, living with both biological parents, and household income. Then, the trimmed list of variables were entered and tested by backward stepwise regression with the above-noted covariates forced in to identify the most parsimonious set of variables associated with risk for a suicidal event. Similarly, variables associated with time to suicidal event from entry into the study were tested in a similar fashion using the Kaplan-Meier method, followed by Cox regressions to identify the most parsimonious set of predictors of time to event.
Results
Events and attempts
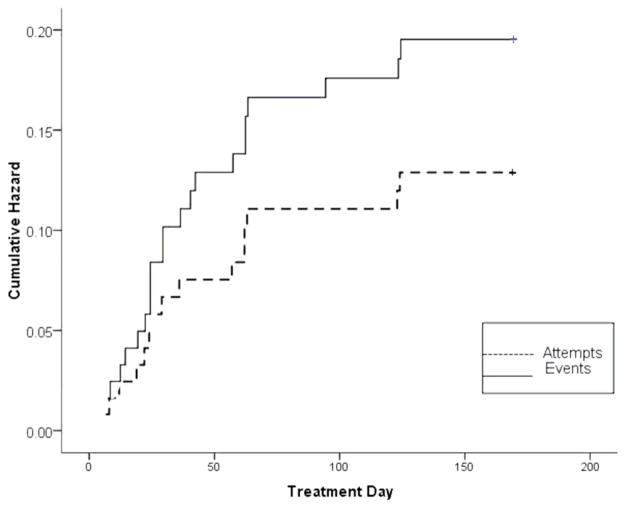
Of the 124 participants enrolled in the study, 24 experienced a suicidal event over the 6 months since entry. Of these 24 participants, 15 made at least one suicide re-attempt. The small number of suicidal events and re-attempts precludes statistical tests comparing types of events, but inspection of the characteristics of re-attempts and other types of suicidal events shows similarity on demographic and clinical baseline variables. The hazard of an event and an attempt were 0.19 and 0.12, respectively, with a mean time to suicidal event from intake of 44.0 days (SE=35.6) and mean time to a re-attempt of 44.8 (SE=37.5) days (see Figure 2). Of the 24 events, 10 occurred within 4 weeks of intake. There was one completed suicide that occurred after a participant had completed treatment, beyond the 6 month window of this report.
Figure 2. Time to Onset of Suicidal Events and Attempts in TASA.
TASA = Treatment of Adolescent Suicide Attempters
Site differences
The demographic characteristics of participants were similar with regard to age and sex across sites, but differed markedly by race (χ2=40.9, df=16, p<.001, See Table 1). There were no overall site differences with regard to rate or time to suicidal events or re-attempts, although there was nearly a 3-fold site variation in the rate of suicidal events (10.7–30.8%).
Table 1.
Descriptive Statistics by Site
| Site | N | Suicidal Events N (%)a | Suicide Attempts N (%)b | Age (yrs, M, SD) | Sex (N [%] male) | Race (N%) | |||
|---|---|---|---|---|---|---|---|---|---|
| White | African- American | Hispanic | Other | ||||||
| 1 | 16 | 4 (25.0) | 3 (18.8) | 15.1 (1.2) | 5 (31.2) | 11 (68.8) | 3 (18.8) | 1 (6.3) | 1 (6.3) |
| 2 | 25 | 4 (16.0) | 2 (8.0) | 15.4 (1.7) | 5 (20.0) | 18 (72.0) | 6 (24.0) | 1 (4.0) | 0 |
| 3 | 28 | 3 (10.7) | 3 (10.7) | 16.2 (1.6) | 5 (17.9) | 9 (32.1) | 3 (10.7) | 12 (42.8) | 4 (14.4) |
| 4 | 29 | 5 (17.2) | 2 (6.9) | 15.9 (1.5) | 6 (20.7) | 24 (82.8) | 1 (3.4) | 3 (10.3) | 1 (3.4) |
| 5 | 26 | 8 (30.8) | 5 (19.2) | 16.0 (1.4) | 7 (26.9) | 21 (80.8) | 3 (11.5) | 2 (7.7) | 0 |
| Total | 124 | 24 (19.4) | 15 (12.1) | 15.8 (1.5) | 28 (22.6) | 83 (66.9) | 16 (12.9) | 19 (15.3) | 6 (4.8) |
Suicide attempt: An act of self-injurious behavior with an inferred or explicit wish to die.
Suicidal event: Completed suicide, attempted suicide, preparatory behavior toward an imminent attempt, or suicidal ideation.
Demographic characteristics and suicidal events
Subsequent suicidal events were not associated with age, race, or ethnic group (see Table 2).
Table 2.
Demographic Characteristics and Risk of Attempt and Suicidal Events
| Suicide Attempts N (%)a | Suicidal Events N (%)b | |||||
|---|---|---|---|---|---|---|
| No | Yes | p | No | Yes | p | |
| N=109 | N=15 | N=100 | N=24 | |||
| Age (yr, M, SD) | 15.8 (1.1) | 15.7 (1) | .81 | 15.7 (1.6) | 16.0 (1.4) | .49 |
| Sex (N, % male) | 24 (22.0) | 4 (26.7) | .68 | 21 (21.0) | 7 (29.2) | .39 |
| Race/Ethnicity (N, %) | .98 | .65 | ||||
| White | 72 (66.1) | 11 (73.3) | 65 (65.0) | 18 (75.0) | ||
| African American | 14 (12.8) | 2 (13.3) | 13 (13.0) | 3 (12.5) | ||
| Hispanic | 17 (15.6) | 2 (13.3) | 16 (16.0) | 3 (12.5) | ||
| Other | 6 (5.5) | 0 | 6 (6.0) | 0 | ||
| Highest education M (SD) | 4.5 (1.2) | 4.3 (1.5) | .46 | 4.5 (1.2) | 4.5 (1.3) | .73 |
Suicide attempt: An act of self-injurious behavior with an inferred or explicit wish to die.
Suicidal event: Completed suicide, attempted suicide, preparatory behavior toward an imminent attempt, or suicidal ideation.
Characteristics of index and past histories of suicidal behavior (Table 3)
Table 3.
Clinical Characteristics of Suicidality and Depressiona
| Suicide Attemptsb | Suicidal Eventsc | |||||
|---|---|---|---|---|---|---|
| No | Yes | p | No | Yes | p | |
| N=109 | N=15 | N=100 | N=24 | |||
| Scale for Suicide Ideation (prior), M (SD) | 23.1 (7.6) | 24.3 | .59 | 23.0 (7.6) | 24.4 (7.3) | .42 |
| Scale for Suicide Ideation (current),d M (SD) | 5.9 (7.3) | 9.5 (10) | <.1 | 5.4 (7.2) | 10.0 (8.8) | .01 |
| No. of previous attempts,e M (SD) | 1.9 (1.9) | 3.8 (3) | .03 | 1.8 (1.6) | 3.8 (3.2) | .007 |
| Hospitalized in past 6 months (N, %) | 70 (71.4) | 8 (72.7) | .93 | 64 (7.1) | 14 (73.7) | .82 |
| Highest lethality attempt,f M (SD) | 2.5 (1.8) | 1.6 (1.1) | .01 | 2.5 (1.8) | 1.8 (1.2) | .03 |
| Age first attempt (years), M (SD) | 15.1 (2) | 14.2 (2.2) | <.1 | 15.1 (1.9) | 14.4 (2.3) | .13 |
| Suicide Intent Scale, M (SD) | 17.7 (5.1) | 18.8 (4.6) | .45 | 17.7 (5.1) | 18.6 (5.0) | .45 |
| CDRS-R, g M (SD) | 49.6 (12.1) | 54.9 (15) | .14 | 49.5 (11.2) | 53.1 (13.1) | .21 |
| BDI, h M (SD) | 21.6 (12.6) | 33.6 (12.2) | .001 | 20.7 (12.5) | 32.4 (11.3) | .001 |
| Duration of depression, M (SD) | 57.1 (57.2) | 73.9 (45.7) | .28 | 55.3 (56.1) | 76.2 (53.3) | .12 |
| No. symptoms of depression, M (SD) | 11.3 (2.8) | 11.7 (11.9) | .64 | 11.2 (2.9) | 11.9 (1.9) | .30 |
| CGAS,i M (SD) | 48.4 (11.1) | 47.9 (11.2) | .86 | 48.9 (11.2) | 46.3 (10.3) | .32 |
| No. comorbid diagnoses, M (SD) | 1.6 (1.2) | 1.6 (1.2) | .94 | 1.6 (1.2) | 1.7 (1.0) | .84 |
N’s vary from 116–124, due to missing data.
Suicide attempt: An act of self-injurious behavior with an inferred or explicit wish to die.
Suicidal event: Completed suicide, attempted suicide, preparatory behavior toward an imminent attempt, or suicidal ideation.
Attempt t=1.67, df=115; event t=2.53, df=116.
Attempt t=2.36, df=15.6; event t=2.94, df=122
Attempt t=2.66, df=25.3; event t=2.28, df=120
CDRS-R=Children’s Depression Rating Scale-Revised
BDI=Beck Depression Inventory; attempt t=3.39, df=116; event t=4.12, df=116
CGAS=Child Global Assessment Scale
Those who experienced a suicidal event had higher levels of suicidal ideation on the SSI at intake, had a greater number of previous attempts, and a lower maximum lethality among previous attempts, the latter two of which also predicted an attempt. Two or more previous attempts and lower maximum lethality of previous attempts predicted early occurrence of suicidal events (Log-rank χ2 ’s from 15.6, p’s < .001).
Characteristics of depressive disorder and events (Table 3)
Those participants who experienced an event showed higher self-reported depression. There was no relationship between experiencing an event and the severity of interview-rated depression, duration of depression, number of interview-rated symptoms, or the symptoms of insomnia or irritability.
Comorbid Diagnoses and events
There were no significant differences in the overall number of comorbid diagnoses (see Table 3), nor were there differences in frequencies of individual comorbid disorders as a function of the occurrence of a suicidal event (data not shown).
Psychological characteristics (Table 4)
Table 4.
Psychological and Family-Environmental Characteristics and Suicidal Behaviora
| Suicide Attemptsb | Suicidal Eventsc | |||||
|---|---|---|---|---|---|---|
| No | Yes | p | No | Yes | p | |
| N=109 | N=15 | N=100 | N=24 | |||
| Aggression, M (SD) | 88.3 (24.4) | 101.4 (29.2) | <.07 | 89.0 (24.7) | 93.2 (27.5) | .48 |
| EASI-Emotion,d M (SD) | 45.2 (9.4) | 49.5 (9.2) | .10 | 45.1 (9.7) | 48.1 (8.2) | .19 |
| EASI-Impulsivity, M (SD) | 62.9 (10.6) | 64.6 (8.5) | .56 | 63.0 (10.6) | 63.4 (9.7) | .88 |
| No. Borderline Symptoms,e M (SD) | 1.6 (2) | 2.5 (2.3) | .11 | 1.6 (2.0) | 2.5 (2.3) | .05 |
| Hopelessness,f M (SD) | 8.9 (6) | 13.7 (5) | .008 | 8.8 (6.0) | 12.6 (5.5) | .008 |
| MASC,g M (SD) | 45.5 (19.4) | 53.8 (10.7) | .10 | 44.3 (17.7) | 55.2 (16.4) | .009 |
| Lives with both biological parents, n, % | 46 (42.2) | 8 (53.3) | .42 | 40 (40.0) | 14 (58.3) | .10 |
| FACESh,i -Adaptability (M, SD) | 39.6 (8.6) | 32.8 (7.9) | .006 | 39.6 (8.6) | 35.3 (8.6) | .03 |
| FACESh,j -Cohesion (M, SD) | 48.6 (12) | 37.4 (7.9) | < .001 | 48.7 (12.3) | 41.1 (9.5) | .006 |
| Sexual abuse (N, %)k | 17 (16.7) | 5 (35.7) | .09 | 14 (15.1) | 8 (34.8) | .03 |
| Physical abuse (N, %)l | 11 (10.8) | 5 (35.7) | .01 | 11 (11.8) | 5 (21.7) | .21 |
N’s vary from 116–124, due to missing data.
Suicide attempt: An act of self-injurious behavior with an inferred or explicit wish to die.
Suicidal event: Completed suicide, attempted suicide, preparatory behavior toward an imminent attempt, or suicidal ideation.
EASI=Emotionality, Adaptability, Sociability, and Impulsivity
Attempt t=1.62, df=122; event t=1.95, df=122
Attempt t=2.71, df=115, event t=2.69, df=116
MASC=Multidimensional Anxiety Scale for Children; Event, t=2.68, df=116
FACES=Family Adaptability and Cohesion Evaluation Scale
Attempt t=2.80, df=116; event t=2.79, df=115
Attempt t=4.59, df=116; event t=2.17, df=115
Event χ2=4.67, df=1
Attempt χ2=6.43, df=1
Baseline self-reported hopelessness, number of borderline personality traits, and severity of anxiety were higher in those who experienced a suicidal event. Self-rated aggression, impulsivity, and emotionality were not associated with the occurrence of a suicidal event.
Family-environmental characteristics (Table 4)
Family constellation at home did not predict the occurrence of an event. A history of reported sexual abuse was associated with a higher risk and earlier onset of an event (Log-rank χ2=5.71, p<.02). A reported history of physical abuse was also associated with an increased risk and earlier occurrence of an attempt (Log-rank χ2= 6.31, p=0.01). High self-rated family adaptability and cohesion were protective against the occurrence of an event.
Impact of treatment on treatment targets and outcome
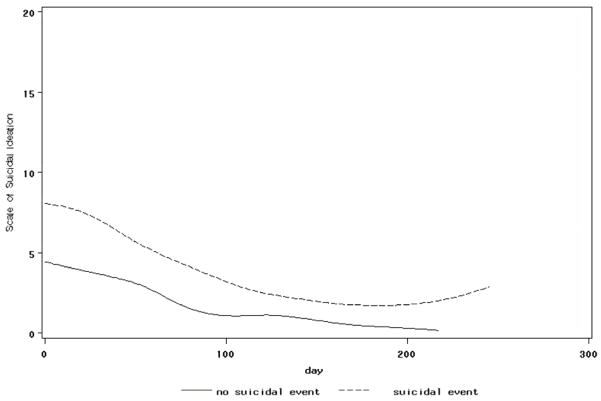
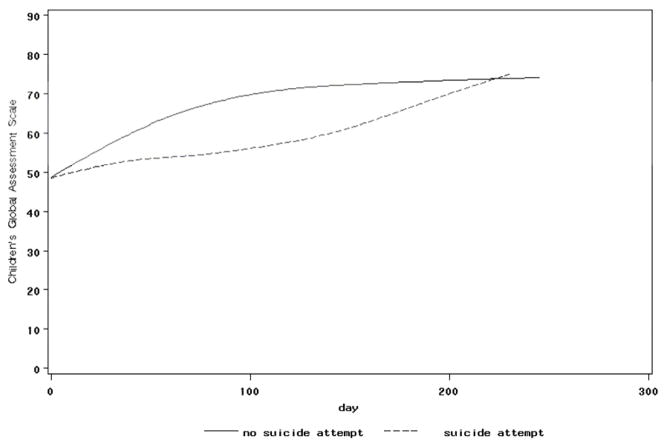
The impact of intervention on baseline predictors of outcome that were treatment targets was examined by conducting a series of random effects regressions, testing for the main effects of time, event status, and event status by time interactions for each treatment target. Effects for time and event status were found in regressions that focused on the BDI, SSI, CGI-S, CGAS, MASC, family cohesion, and family adaptability, meaning that these measures of symptomatology and functioning improved over time, and were higher at intake in those who eventually experienced an event. Event by time interactions were found for the SSI with regard to events (F [1,377]=4.40, p=.037), and the CGAS with regard to attempts (F [1,352]=5.74, p=.017); see Figures 3 and 4). These results mean that those participants who experienced a suicidal event had a slower decline in suicidal ideation than those who did not, whereas those who made a suicide attempt showed slower improvement in their functional status.
Figure 3. Mean SSI by Suicidal Event Group.
SSI = Scale of Suicide Ideation
Figure 4. Mean CGAS by Suicide Attempt Group.
CGAS = Children’s Global Assessment Scale
Logistic regression
The most parsimonious set of predictors of an event were higher parental income (OR=2.6, 95% CI = 1.03, 6.8), site (OR=4.5, 95% CI 1.1, 18.5), a history of sexual abuse (OR=18.2, 95% CI 2.5 130.6), and lower lethality of previous attempts (OR=0.5, 95% CI 0.3, 0.9).
Time to event
Predictors of earlier time to onset of a suicidal event, using Cox regression were: higher income (OR=2.2, 95% CI 1.0, 4.7), white race (OR=2.6, 95% CI 1.1–5.0), site (OR=4.6, 95% CI 1.4, 15.4), the number of previous suicide attempts (OR=1.5, 95% CI 1.1, 1.9), reported history of sexual abuse (OR=4.4, 95% CI 1.1, 18.0). Both lower maximum lethality of previous attempt (OR=0.6, 95% CI 0.4, 0.96), and higher family cohesion were protective against the occurrence of an event (OR=.94, 95%CI 0.7–1.0).
Type of treatment and outcome
While the majority of youth received a combination of medication and psychotherapy (n=93), 31 participants were in one of the monotherapy groups, either pharmacotherapy (n=14) or CBT (n=17). The rate of suicidal events was higher in the combination group (22/93 [23.7%] vs. 2/31 [6.5%] Fisher’s, p<.04). However, those participants who received combination treatment were in a higher risk category for repeated suicide events, as they showed higher interview and self-rated depression scores, higher hopelessness, greater number of previous attempts, a higher rate of psychiatric hospitalization in the 6 months prior to treatment, and lower levels of functioning. Logistic regression was conducted to evaluate the relationship between monotherapy vs. combined therapy, and after controlling for these baseline differences, there was no differential effect of treatment type on suicidal outcomes.
Discussion
In this open feasibility treatment study of depressed adolescent suicide attempters, strong clinical predictors of experiencing a suicidal event were high levels of suicidal ideation and self-reported depression, a history of maltreatment, two or more previous attempts, lower lethality of the index attempt, and lower levels of family cohesion. When participants showed a slower reduction in suicidal ideation, they were also more vulnerable to experiencing a suicidal event. Similar, but not identical factors predicted a re-attempt. The hazard of an event and attempt over 6 months post-intake were 0.19 and 0.12, respectively, with a median time to an event or attempt of around 6 weeks. Approximately 40% of events occurred within 4 weeks of entrance into the study. The research and clinical implications of this study will be discussed after putting them in the context of its limitations and strengths.
Because this study was in large part a non-randomized trial in which participants chose their treatment, we cannot address questions of the efficacy of our intervention, or its component parts. On the other hand, because our sample received relatively standard and similar treatments, our assessment of predictors for re-attempt and for suicidal events may be more clinical meaningful than in most naturalistic longitudinal studies, in which participants may receive interventions of varying intensity and quality. We also are limited by the relatively small number of participants, events, and attempts, although homogeneity in entry and outcome criteria does allow us to draw some conclusions about depressed adolescent suicide attempters. While we endeavored to cover relevant domains to suicide risk in our assessment, we have relatively little specific information about some salient domains, such as details about maltreatment, inter-current stressful life events, and strategies of emotion regulation.1;3;4;8 Finally, significant site effects emerged that could be due either to differences in some of the above-noted characteristics, or in the implementation of treatment.
Nevertheless, this study has some unique characteristics, despite the above-noted limitations. We engaged a very difficult-to-treat population, the characteristics of which usually lead to exclusion from clinical trials, with a relatively high rate of follow-up. While we cannot determine whether the treatment is efficacious, we can identify predictors of response that should be able to guide future treatment development.
Our sample, treated for 6 months, had hazards of experiencing a suicidal event or a suicide attempt of 0.19 and 0.12, respectively, with a median time to a suicidal event of around 44 days. In one 6-month follow-up study of hospitalized adolescents, the hazards of re-attempt in adolescents admitted for an attempt or for significant suicidal ideation were 0.17 and 0.27, respectively.6 The 6-month hazard of suicidal events in those depressed inpatients with a history of either suicidal ideation with a plan or an attempt was 0.69 (SE=0.15). In a re-analysis of Goldston et al.’s7 five year follow-up of formerly hospitalized adolescents, the hazard of a re-attempt at 6 months and one year post-hospital discharge for those with a history of at least one previous attempt and a mood disorder were 0.20 and 0.30, respectively.34 Our results, in a comparable sample, many of whom had recently been discharged from the hospital, compare favorably to the outcome for both of these samples. This was true even for the highest risk group that received combination treatment, with a 6-month rate of events of 23.6%. While such comparisons are promising, they only suggest that our intervention may be helpful in reducing risk for recurrent suicidal behavior.
On the other hand, 10/24 of the suicidal events occurred within 4 weeks of intake into treatment, meaning that many events occurred prior to a time when an adequate “dose” of psychotherapy or pharmacotherapy could be delivered. The early timing of these events, which has been reported in other similar samples, suggest the importance of front-loaded intervention strategies that are most likely to reduce risk of recurrent suicidal behavior, through a careful elaboration of a safety plan and coping strategies for likely precipitants for suicidal behavior.35
Our findings that high self-reported depression, high ideation at the beginning of treatment, high hopelessness, and a history of multiple attempts predicted a suicidal event are consistent with other reports.1;6;7;35;36 Unlike other studies, we did not find that comorbid disruptive disorders were related to increased risk for attempt, perhaps because participants with significant conduct symptoms that could not be managed in the context of the treatment were excluded.1;4 Self-rated depression was a much stronger predictor of re-attempt and occurrence of an event than interview-rated depression, perhaps because this gap between self-report and interview may be indicative of poor distress tolerance and impaired emotion regulation.37
Our finding of an association between child maltreatment and risk for recurrent suicidal behavior has previously been well-documented.1;4 Collectively, these results reinforce the importance of the assessment and management of trauma, and the salience of a safe environment for the suicidal child.
Conversely, higher family cohesion and adaptability were protective against future events. Other studies have found that positive relationships with a parent, parental monitoring and supervision, a positive connection with school, and a pro-social peer group were protective against the occurrence of suicide attempts, even in the face of other risk factors, including abuse.3;38 Enhancement of positive parenting and adaptive family coping may be useful in preventing reattempts.
Some of the findings were unexpected. In many studies, ethnic minority status and economic distress are predictors of suicidal ideation and behavior,39 whereas in this cohort, higher income and white race were associated with earlier time to a suicidal event. Although these results persisted after controlling for site, it is possible that these associations may be a function of differences in site ethnic make-up and performance. The sites with the highest proportion of white participants had the highest rates of suicidal events. Those with lower income were more likely to be lost to follow-up, which may have contributed to an underestimate of the rate of suicidal events in that subgroup. The association between lower lethality of suicide attempts and recurrent suicidal behavior is also counterintuitive, but those who have engaged in multiple suicide attempts tend to make more impulsive attempts, which in other studies have been shown to be inversely correlated with lethality.36;40 These findings confirm that depressed youth with a history of a suicide attempt are at high risk for recurrent suicidal behavior. Salient therapeutic targets may include the development of distress tolerance and improved emotion regulation, targeting the residua of childhood trauma, and enhancement of protective elements in family, school, and social environments. While this is primarily an open study of the impact of intensive and specialized pharmacological and/or psychotherapeutic treatment, the observed re-attempt and suicide event hazard rates compare favorably to reports in the literature in similar samples, suggesting a potential therapeutic benefit for these interventions. Since a significant proportion of suicidal events tended to cluster shortly after intake, before the benefit of any of these interventions may be evident, an emphasis on safety planning and on increasing the intensity of the therapeutic contact early in treatment may be warranted.
Acknowledgments
Funding/Support: Funded by the National Institute of Mental Health through cooperative agreement grants MH66750 (Duke University Medical Center), MH66769 (Johns Hopkins University), MH66762 (New York State Psychiatric Institute), MH66775 (University of Pittsburgh), and MH66778 (University of Texas Southwestern Medical Center).
Footnotes
This article is the subject of an editorial by Dr. Garry Walter in this issue.
Disclosure: Dr. Greenhill was a consultant to Pfizer and served as chairman of DSMB for pediatric ziprasidone trials; received research grants from NIMH, Otsuka/Bristol Myers-Squib, Johnson & Johnson.
Dr. Emslie received research support Biobehavioral Diagnostics Inc., Forest Laboratories, Shire, and Somerset; has been a consultant for Biobehavioral Diagnostics Inc., Eli Lilly, Forest Laboratories Inc, Pfizer Inc., Shire, Validus Pharmaceuticals, and Wyeth Pharmaceuticals.
Dr. Bukstein has consulted for Quintiles CME, has served on the speakers’ bureau of Quintiles CME, has received research and/or education funding from Shire Pharmaceuticals, Ortho-McNeil-Janssen, and Quintiles CMS, and has received book royalties from Routledge.
Dr. Walkup receives research support from the NIH, SAMHSA, and the Tourette Syndrome Association. He received free medication and placebo from Eli Lilly, Pfizer and free medication from Abbott for NIMH funded clinical trials. He has received honoraria from Eli Lilly in 2006 and from Pfizer in 2003 and 2005 and from the Tourette Syndrome Association in 2007–8. He also provided consultation to defense council on behalf of GlaxoSmithKline (Paxil) in 2007.
Dr. Coffey has received research support from NIMH, Boehringer Ingelheim, Bristol Myers Squibb, Novartis, and Tourette Syndrome Association, and has been advisor to Novartis and Jazz Pharmaceuticals.
Dr. Posner has received only research support from the following pharmaceutical companies, as part of an effort to help execute the FDA suicidality mandates/requests: Amgen, Astra Zeneca Pharmaceuticals, Forest Laboratories, GlaxoSmithKline, i3 Research, Eli Lilly, Johnson & Johnson, H. Lundbeck A/S, Medtronic, Merck & Co., Inc., Next Wave Pharmaceuticals, Novo Nordisk A/S, Orexigen Therapeutics, Otsuka Pharmaceuticals, Pfizer, Roche, Sanofi-Aventis, Schering-Plough Corporation, Schwarz Biosciences, Inc./UCB, Sepracor, Inc., Takeda Pharmaceutical Company, Valeant Pharmaceuticals, Vivus, Inc., and Wyeth Research.
Dr. March receives research support from NIMH and NARSAD; is a consultant or scientific advisor to Pfizer, Lilly, Wyeth, GSK, and MedAvante; receives research support from Pfizer and Lilly; has equity holdings in MedAvante; receives study drug for NIMH-funded studies from Lilly and Pfizer.
Dr. Riddle receives research funding from NIMH, has served as DSMB member for NICHD and Johnson & Johnson, has been a consultant/scientific advisor to Shire and Jazz Pharmaceuticals.
Dr. Wagner held stock in Johnson & Johnson, within NIH limit, and has since divested.
Dr. Curry receives speaking fees from the REACH Institute.
Dr. Wells receives speaking fees from the REACH Institute.
The other authors report no conflicts of interest.
Publisher's Disclaimer: Disclaimer: The opinions and assertions contained in this report are the private views of the authors, and are not to be construed as official or as reflecting the views of the National Institute of Mental Health, the National Institutes of Health, or the Department of Health and Human Services.
Reference List
- 1.Bridge JA, Goldstein TR, Brent DA. Adolescent suicide and suicidal behavior. J Child Psychol Psychiatry. 2006;47:372–394. doi: 10.1111/j.1469-7610.2006.01615.x. [DOI] [PubMed] [Google Scholar]
- 2.Goldsmith SK, Pellmar TC, Kleinman AM, Bunney WE, editors. Committee on Pathophysiology & Prevention of Adolescent & Adult Suicide, Institute of Medicine. Reducing Suicide: A National Imperative. Washington, DC: The National Academies Press; 2002. [PubMed] [Google Scholar]
- 3.Borowsky IW, Ireland M, Resnick MD. Adolescent suicide attempts: Risks and protectors. Pediatrics. 2001;107:485–493. doi: 10.1542/peds.107.3.485. [DOI] [PubMed] [Google Scholar]
- 4.Gould MS, Greenberg T, Velting DM, Shaffer D. Youth suicide risk and preventative interventions: A review of the past 10 years. J Am Acad Child Adolesc Psychiatry. 2003;42:386–405. doi: 10.1097/01.CHI.0000046821.95464.CF. [DOI] [PubMed] [Google Scholar]
- 5.Oquendo MA, Stanley B, Ellis SP, Mann JJ. Protection of human subjects in intervention research for suicidal research. Am J Psychiatry. 2004;191:1558–1563. doi: 10.1176/appi.ajp.161.9.1558. [DOI] [PubMed] [Google Scholar]
- 6.Brent DA, Kolko DJ, Wartella ME, Boylan MB, Moritz G, Baugher M, Zelenak JP. Adolescent psychiatric inpatients’ risk of suicide attempt at 6-month follow-up. J Am Acad Child Adolesc Psychiatry. 1993;32(1):95–105. doi: 10.1097/00004583-199301000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Goldston DB, Daniel SS, Reboussin DM, Reboussin BA, Frazier PH, Kelley AE. Suicide attempts among formerly hospitalized adolescents: A prospective naturalistic study of risk during the first 5 years after discharge. J Am Acad Child Adolesc Psychiatry. 1999;38:660–671. doi: 10.1097/00004583-199906000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Lewinsohn PM, Rohde P, Seeley JR. Adolescent suicidal ideation and attempts: Prevalence, risk factors, and clinical implications. Clin Psychol Sci Prac. 1996;3:25–46. [Google Scholar]
- 9.Posner K, Oquendo M, Stanley B, Davies M, Gould M. Columbia Classification Algorithm of Suicide Assessment (C-CASA): Classification of suicidal events in the FDA’s pediatric suicidal risk analysis of antidepressants. Am J Psychiatry. 2007;164:1035–1043. doi: 10.1176/appi.ajp.164.7.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes CW, Emslie GJ, Crismon ML, Posner K, Birmaher B, Ryan N, Jensen P, Curry J, Vitiello B, Lopez M, Shon SP, Pliszka SR, Trivedi MH. Texas Consensus Conference Panel on Medication Treatment of Childhood Major Depressive Disorder: Texas Children’s Medication Algorithm Project: Update from Texas consensus conference panel on medication treatment of childhood major depressive disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:667–686. doi: 10.1097/chi.0b013e31804a859b. [DOI] [PubMed] [Google Scholar]
- 11.Brown GK, Have TT, Henriques GR, Xie SX, Hollander JE, Beck AT. Cognitive therapy for the prevention of suicide attempts. J Am Med Assoc. 2005;294:563–570. doi: 10.1001/jama.294.5.563. [DOI] [PubMed] [Google Scholar]
- 12.Wells K, Curry JF. TADS Family Manual. Durham, NC: Duke University; 2000. [Google Scholar]
- 13.Curry JF, Wells K, Brent D, Clarke G, Rohde J, Albano AM, Reinecke MA, Benazon A, March J. TADS Adolescent CBT Manual. Durham, NC: Duke University; 2000. [Google Scholar]
- 14.Brent DA, Emslie GJ, Clarke GN, Wagner KD, Asarnow J, Keller MB, Vitiello B, Ritz L, Iyengar S, Abebe K, Birmaher B, Ryan N, Kennard B, Hughes C, Debar L, McCracken J, Strober M, Suddath RL, Spirito A, Leonard H, Melhem NM, Porta G, Onorato M, Zelazny J. Switching to venlafaxine or another SSRI with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression: The TORDIA randomized control trial. JAMA. 2008;299:901–913. doi: 10.1001/jama.299.8.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brent DA, Poling K. Cognitive Therapy Manual for Depressed and Suicidal Youth. 1997 [Google Scholar]
- 16.Comtois KA, Linehan MM. Psychosocial treatments of suicidal behaviors: A practice-friendly review. J Clin Psychol. 2006;62:161–170. doi: 10.1002/jclp.20220. [DOI] [PubMed] [Google Scholar]
- 17.Emslie GJ, Mayes T, Greenhill L, March J, Vitiello B, Walkup J. Pharmacological management of depressed suicidal adolescents. In preparation. [Google Scholar]
- 18.Stanley B, Brown G, Brent D, Wells K, Poling K, Kennard B, Wagner A, Curry J, Cwik M, Goldstein T, Klomek-Brunstein A, Barnett S, Daniel S. Cognitive Behavior Therapy for Suicide Prevention (CBT-SP): Treatment model, feasibility and acceptability. J Am Acad Child Adolesc Psychiatr. doi: 10.1097/CHI.0b013e3181b5dbfe. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meltzer HY, Okayli G. Reduction of suicidality during clozapine treatment of neuroleptic-resistant schizophrenia: Impact on risk-benefit assessment. Am J Psychiatry. 1995;152:183–190. doi: 10.1176/ajp.152.2.183. [DOI] [PubMed] [Google Scholar]
- 20.Kessler RC, Borges G, Walters EE. Prevalence of and risk factors for lifetime suicide attempts in the national comorbidity survey. Archives of General Psychiatry. 1999;56:617–626. doi: 10.1001/archpsyc.56.7.617. [DOI] [PubMed] [Google Scholar]
- 21.Vitiello B, Brent D, Greenhill L, Emslie G, Wells K, Walkup J, Stanley B, Bukstein O, Kennard B, Compton S, Coffey B, Cwik M, Posner K, Wagner A, March J, Riddle M, Goldstein T, Curry J, Capasso L, Mayes T, Shen S, Gugga S, Turner JB, Barnett S, Zelazny J. Depressive symptoms and clinical status during the Treatment of Adolescent Suicide Attempters (TASA) Study. J Am Acad Child Adolesc Psychiatr. doi: 10.1097/CHI.0b013e3181b5db66. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mann JJ, McBride PA, Brown RP, Linnoila M, Leon AC, DeMeo M, Mieczkowski T, Myers JE, Stanley M. Relationship between central and peripheral serotonin indexes in depressed and suicidal psychiatric inpatients. Archives of General Psychiatry. 1992;49:442–446. doi: 10.1001/archpsyc.1992.01820060022003. [DOI] [PubMed] [Google Scholar]
- 23.Beck AT, Schuyler D, Herman I. Development of suicidal intent scales. In: Beck AT, Lettieri DJ, Resnick HLP, Bowie MD, editors. The Prediction of Suicide. Charles Press; 1974. pp. 45–55. [Google Scholar]
- 24.Beck AT, Kovacs M, Weissman A. Assessment of suicidal intention: The Scale for Suicide Ideation. J Consult Clin Psychol. 1979;47(2):343–352. doi: 10.1037//0022-006x.47.2.343. [DOI] [PubMed] [Google Scholar]
- 25.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 26.Poznanski EO, Freeman LN, Mokros HB. Children’s Depression Rating Scale - Revised. Psychopharmacol Bull. 1984;21:979–989. [Google Scholar]
- 27.Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review. 1988;8:77–100. [Google Scholar]
- 28.Beck AT, Weissman A, Lester D, Trexler L. The measurement of pessimism: The Hopelessness Scale. J Consult Clin Psychol. 1974;42(6):861–865. doi: 10.1037/h0037562. [DOI] [PubMed] [Google Scholar]
- 29.March JS, Parker JDA, Sullivan K, Stallings P, Conners K. The Multidimensional Anxiety Scale for Children (MASC): Factor structure, reliability, and validity. J Am Acad Child Adolesc Psychiatry. 1997;36:554–565. doi: 10.1097/00004583-199704000-00019. [DOI] [PubMed] [Google Scholar]
- 30.Buss AH, Perry M. The Aggression Questionnaire. J Pers Soc Psychol. 1992;63:452–459. doi: 10.1037//0022-3514.63.3.452. [DOI] [PubMed] [Google Scholar]
- 31.Buss AH, Plomin R. Temperament: Early Developing Personality Traits. Hillsdale, NJ: Lawrence Erlbaum; 1984. [Google Scholar]
- 32.Wagner AW, Linehan MM. Relationship between childhood sexual abuse and topography of parasuicide among women with borderline personality disorder. J Pers Disorders. 1994;8:1–9. [Google Scholar]
- 33.Olsen DH, Portner J, Lavee Y. Family Adaptability and Cohension Evaluation Scales (FACES-II) University of Minnesota Press; 1985. [Google Scholar]
- 34.Goldston D. Personal communication. 2008 [Google Scholar]
- 35.Brent D, Emslie G, Clarke G, Asarnow JR, Spirito A, Ritz L, Vitiello B, Iyengar S, Birmaher B, Ryan N, Zelazny J, Onorato M, Kennard B, Mayes T, Debar L, McCracken J, Strober M, Suddath R, Leonard H, Porta G, Keller M. Predictors of spontaneous and systematically assessed suicidal adverse events in the Treatment of SSRI-Resistant Depression in Adolescents (TORDIA) Study. Am J Psychiatry. 2009;166:418–426. doi: 10.1176/appi.ajp.2008.08070976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miranda R, Scott M, Hicks R, Wilcox HC, Harris Munfakh JL, Shaffer D. Suicide attempt characteristics, diagnoses, and future attempts: Comparing multiple attempters to single attempters and ideators. J Am Acad Child Adolesc Psychiatry. 2008;47:32–40. doi: 10.1097/chi.0b013e31815a56cb. [DOI] [PubMed] [Google Scholar]
- 37.Stanley B, Wilson ST. Heightened subjective expereience of depressed symptomatology in borderline personality disorder. J Pers Disorders. 2006;20:307–318. doi: 10.1521/pedi.2006.20.4.307. [DOI] [PubMed] [Google Scholar]
- 38.Lynskey MT, Fergusson DM. Factors protecting against the development of adjustment difficulties in young adults exposed to childhood sexual abuse. Child Abuse and Neglect. 1997;21:1177–1190. doi: 10.1016/s0145-2134(97)00093-8. [DOI] [PubMed] [Google Scholar]
- 39.Hawton K, Harriss L, Hodder K, Simkin S, Gunnell D. The influence of the economic and social environment on deliberate self-harm and suicide: An ecological and person-based study. Psychol Med. 2001;31:827–836. doi: 10.1017/s0033291701003993. [DOI] [PubMed] [Google Scholar]
- 40.Baca-Garcia E, Diaz-Sastre C, Basurte E, Prieto R, Ceverino A, Saiz-Ruiz J, de Leon J. A prospective study of the paradoxical relationship between impulsivity and lethality of suicide attempts. J Clin Psychiatry. 2001;62:560–564. doi: 10.4088/jcp.v62n07a11. [DOI] [PubMed] [Google Scholar]