Abstract
Nitric oxide (NO) protection is crucial against angiotensin II (ANG II) mediated vasoconstriction in postnatal preglomerular resistance vessels. While whole kidney nitric oxide synthase (NOS) is developmentally regulated, NOS regulation in developing renal resistance vessels is unknown. The hypothesis was NOS expression and function in developing afferent arterioles is regulated by ANG II through AT1 and AT2 receptors. Afferent arterioles from porcine kidneys, ages newborn, 7, 21 days, and adult, were dissected using a polybead perfusion technique. Dissected afferent arterioles were treated with ANG II and with either the AT1 receptor inhibitor candesartan, or the AT2 receptor inhibitor PD 123319, and evaluated for NOS isoform expression and NOS enzymatic activity. While NOS activity and neuronal NOS (nNOS) expression were greater in the newborn than in the adult, endothelial NOS (eNOS) expression was greater in the adult. ANG II increased NOS activity and eNOS expression at all ages, but nNOS expression only in developing afferents. AT1 and AT2 receptor blockade significantly attenuated NOS activity and eNOS expression at all ages, but nNOS expression only in developing afferents. Conclusion: ANG II regulates nNOS and eNOS expression and NOS activity in afferent arterioles of the developing kidney via AT1 and AT2 receptors.
Introduction
Newborn renal hemodynamics is characterized by low renal blood flow (RBF) and glomerular filtration rate (GFR), which both progressively increase with maturation (1-3). During maturation, the immature kidney experiences an 86% decrease in renal vascular resistance (RVR) and the amount of cardiac output that the kidney receives increases over eight-fold as RBF increases (1,4). The predominant factor contributing to the newborn's low RBF is high RVR (1-3). Although structural and physical factors play a role in the change in RVR and RBF seen in the developing kidney, an increase in functional vasoactive agents is the main cause for the neonate's enhanced RVR (1-4).
In the adult kidney, the primary site of RVR in the preglomerular vessels occurs in the afferent arterioles (5). Studies involving various species have suggested RVR is further enhanced in the afferent arterioles of the newborn kidney causing functional decreases in RBF, GFR, and filtration fraction, thus highlighting the hemodynamic significance of enhanced vascular resistance in the newborn's afferent arterioles (1,6,7). The major vasoactive agent that causes the newborn's elevated RVR is the renin-angiotensin system (RAS) (8-11). Many components of the RAS are developmentally regulated in the immature preglomerular resistance vessels. The vasoactive affects of angiotensin II (ANG II) are mediated in the preglomerular vasculature by two receptor subtypes, the AT1 and AT2 receptors, both of which undergo developmentally regulated expression (1,11-14). However, the functions of the two ANG II receptors in the immature kidney have yet to be fully identified.
The two nitric oxide synthase (NOS) isoforms contributing nitric oxide (NO) to the newborn's renal hemodynamic state under physiological conditions are endothelial NOS (eNOS) and neuronal NOS (nNOS), both constitutively expressed within the immature kidney (14-16). Various studies have shown that NO plays a much more pronounced role in the neonate's renal hemodynamics, than in the adult's, and NO counter-balances the highly activated RAS protecting the immature kidney from the deleterious effects of adverse perinatal events that lead to vasomotor acute renal failure (8,15,17-20). In whole kidney models, developmental patterns of eNOS and nNOS are different, suggesting the isoforms have different regulatory factors and functional contributions in the immature kidney (15,16). However, studies designed to investigate the relationship between NO and ANG II in the preglomerular resistance vasculature have been limited to adult models, thus leaving the neonate unexamined.
The objective of the study here was to determine the role of ANG II as a regulator of eNOS and nNOS expression and function of NO production in the immature, as compared to the mature, preglomerular resistance vessels, primarily the afferent arteriole. The hypothesis of the study is that in the immature afferent arteriole, NOS expression and function are upregulated by ANG II through activation of the AT1 and AT2 receptors.
Materials and Methods
Animals
All experiments involving animal usage fulfilled AVMA guidelines and were approved by the Institutional Animal Care and Use Committee (IACUC) at Eastern Virginia Medical School. The animal study protocol was in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
These experiments utilized afferent arterioles obtained from kidney slices from ages representative of the developmental spectrum including newborns, 7, 21 day-old piglets and adults (NB, 7D, 21D, and AD, respectively) (n = 10 per age group). All experiments were performed on mixed breed swine obtained from the same local vendor (Smithfield, VA). Left kidneys of preweanling piglets (newborns and 7 day-olds) were harvested on arrival. Left kidneys of weaned piglets (21 day-olds) and adults greater than 70 days of age were harvested 24 hours after arrival to allow for dietary and climatic adjustment.
Preparation
Animals were initially anesthetized with intramuscular ketamine (adults 10 mg/kg; piglets 5 mg/kg), followed by a sacrificial intravenous euthasol infusion (50 mg/kg) (Delmorva Laboratories, Midlothian, VA). The left kidney was exposed through a retroperitoneal flank incision and a catheter was placed in the proximal renal artery for intrarenal perfusion.
Microdissection of renal afferent arterioles
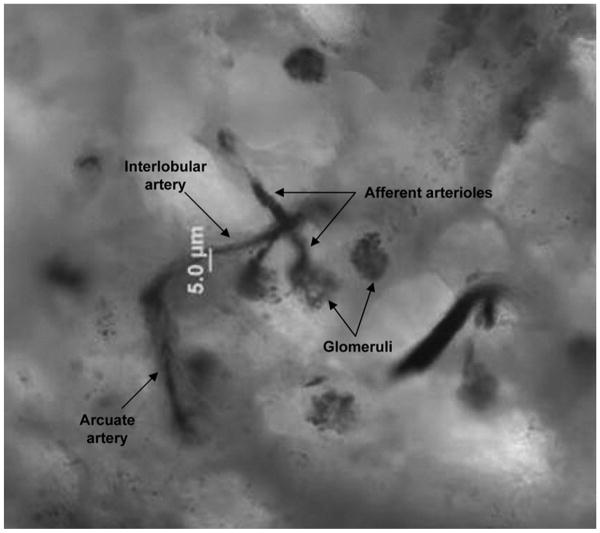
The techniques here are modified from previous publication (21,22). Left kidneys were initially perfused with PBS (Invitrogen, Carlsbad, CA), followed by perfusion of 3 ml of PBS containing polybead polystyrene blue-dyed microspheres 0.2-10 um in diameter (Polysciences, Warrington, PA). Kidneys were excised, decapsulated, and collecting systems were removed. Kidneys were sectioned into 500 um coronal slices (Maclwain Chopper, Mickle Laboratory, Philadelphia, PA) (Fig. 1) and digested with 2 mg/ml collagenase (Sigma). Digested slices were placed in DMEM (Invitrogen) containing protease inhibitors and 0.1 μM candesartan (Astra Zeneca, Sweden) for AT1 receptor inhibition (AT1X), 0.1 μM PD 123319 (Sigma) for AT2 receptor inhibition (AT2X), or medium alone (control and ANG II). Kidney slices were maintained at 4°C during microdissection on the dissecting microscope stage. Manual dissection of afferent arterioles included using dissecting forceps, iris scissors, and 33 gauge stainless steel needles. For optimal expression and enzymatic activity results, dissecting time was restricted to 1.5 hours, as determined by preliminary experiments here (not shown) and by Mattson and Fu (21). During dissection, 20 mm of microvessel was obtained per sample. Dissected afferent arterioles were obtained near the juxtaglomerular axis to ensure homogenous consistency between all samples analyzed.
Fig. 1.
Magnification (50×) of a porcine 7 day-old corticomedullary kidney slice stained with perfused blue-dyed polybeads.
mRNA and protein expression assay
Dissected microvessels were incubated in Krebs buffer (Sigma) for either 1 hour (mRNA study) or for 8 hours (protein study) with 0.1 μM ANG II (Sigma), 0.1 μM candesartan and 0.1 μM ANG II, 0.1 μM PD 123319 and 0.1 μM ANG II, or medium alone. At the conclusion of the incubation period, microvessels were homogenized by sonication, mRNA was isolated using the RNeasy Kit (Qiagen, Valencia, CA), and protein was used for western blots.
Real-time RT-PCR
Isolated mRNA was subject to real-time RT-PCR, as previously described (13). In brief, the LightCycler (Roche, New York, NY) was used for all real-time PCR reactions utilizing the LightCycler FastStart DNA Master SYBR Green I amplification kit (Roche). Polymerase amplification was carried out for 40 cycles. At the onset of expression experiments, PCR products were sequenced to ensure product specificity. Primers (Invitrogen) used were specific for nNOS, eNOS, and 18s rRNA. Primers sequences and Genbank accession numbers are described elsewhere (13). 18s rRNA was used as internal control for gene expression of NOS isoforms. The expression of 18s rRNA is an effective internal reference because it does not undergo significant regulation in the postnatal immature kidney.
Western blot analysis
Western blots were performed on afferent arteriole homogenates, as previously described (15,16). In brief, 10 μg of arteriole protein homogenates, as measured by Bradford Protein Assay (Bio-Rad, Hercules, CA), were separated by SDS-PAGE, blotted onto nitrocellulose (Hy-Bond, Amersham, Arlington Heights, IL), and blocked with nonfat dry milk. Blots were incubated with antibodies specific for porcine eNOS (Santa Cruz, Santa Cruz, CA) and nNOS (Abcam, Cambridge, MA), followed by incubation with horseradish-peroxidase-conjugated secondary antibody (Abcam) and detected by enhanced chemiluminescence (Amersham). Samples were normalized to β-tubulin (Santa Cruz).
NOS activity assay
NOS activity was measured as the conversion of L-3H-arginine to L-3H-citrulline in afferent arterioles, as described previously (23). Briefly, arterioles were incubated in buffer containing L-3H-arginine for 1 hour. Included in the incubation buffer were 0.1 μM ANG II, 0.1 μM candesartan with 0.1 μM ANG II, or 0.1 μM PD 123319 with 0.1 μM ANG II. At the conclusion of the incubation period, the amount of L-3H-arginine converted to L-3H-citrulline in buffer was measured (after separation by Dowex resin (Bio-Rad)). Data are expressed as picomoles of L-3H-arginine converted to L-3H-citrulline per minute per mg of protein minus any activity not inhibited by NOS inhibitors, with adjustment for background levels. Background levels were determined in heat-inactivated samples, and represent free tritium or noncationic tritiated species not separated by Dowex resin. Total counts available for conversion were determined by 100% standard prepared without Dowex resin purification. Pelletized microvessels removed from the NOS activity assay were used in a Bradford Protein Assay (Bio-Rad), to assess the amount of microvessels in each sample.
Statistical analysis
Results obtained for NOS expression and enzymatic activity are expressed as means ± SEM. For multiple comparisons between various ages and effect of treatment, a 2-way ANOVA with Bonferroni's post-test was performed using GraphPad Prism version 4.00 for Windows (GraphPad, San Diego, CA). Differences were considered significant at P < 0.05.
Results
Successful staining of the preglomerular vasculature using polybead perfusion is illustrated in Fig. 1.
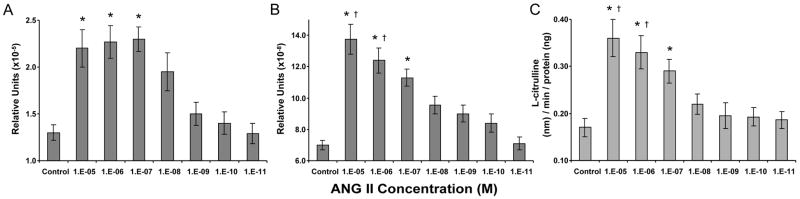
We initially ran an ANG II dose dependent trial on new born dissected afferent arterioles to determine the optimal concentration of ANG II to use in subsequent NOS experiments. eNOS and nNOS mRNA expression and NOS enzymatic activity progressively increased with increased ANG II concentration, with the exception of eNOS mRNA expression, which reached maximum expression at concentration 10-7 M with no further increase in expression with increased ANG concentration (Fig. 2). NOS activity and mRNA expression demonstrated significant reduction when ANG II concentrations were below 10-7 M.
Fig. 2.
ANG II dose response trial in newborn afferent arterioles regarding NOS mRNA expression. A.) eNOS; B.) nNOS; C.) NOS enzymatic activity. *P<0.05, vs. control, 1e-9, 1e-10, 1e-11; †P<0.05, vs. 1e-8.
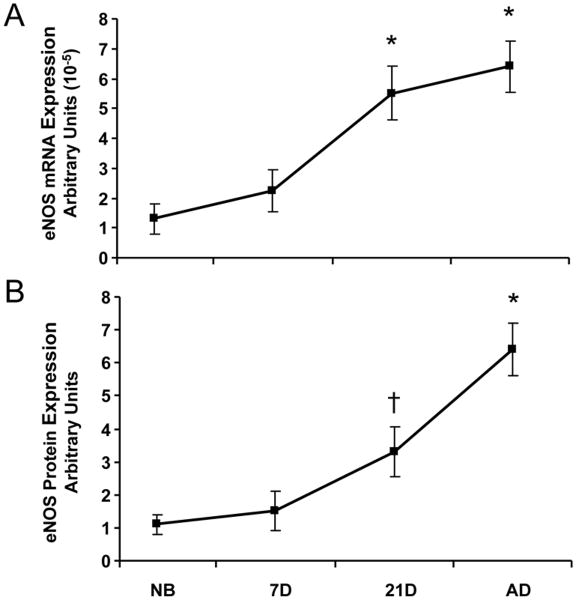
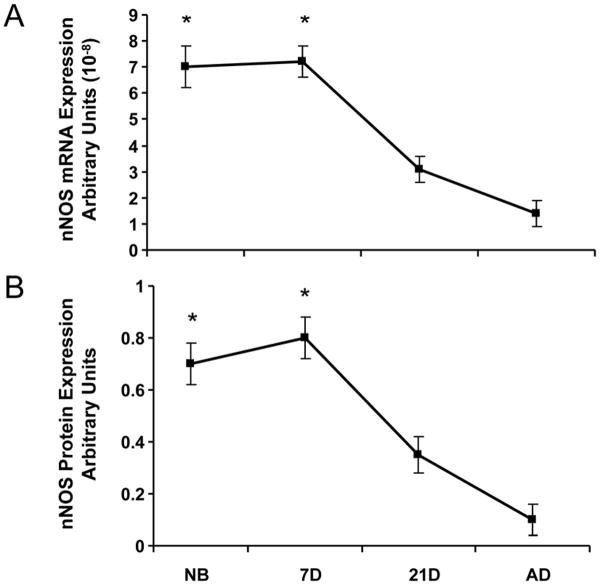
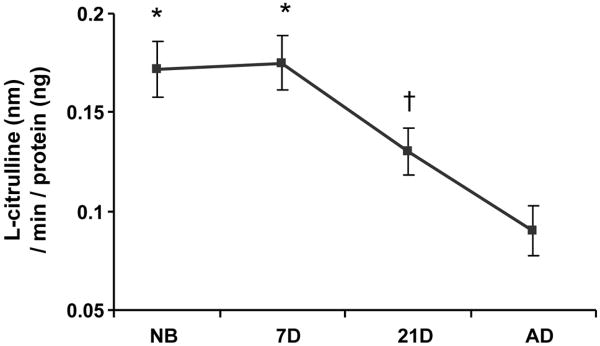
Basal expression of eNOS mRNA and protein in afferent arterioles demonstrated similar patterns. eNOS mRNA and protein expression were minimal immediately after birth in the newborn and 7 day-old, but with continued vascular maturity, NOS expression progressively increased in the 21 day-old before reaching maximal levels in the adult afferent arteriole (Fig. 3). In contrast to the observed pattern for eNOS expression, nNOS mRNA and protein basal expression were enhanced in the period immediately after birth in the newborn and 7 day-old afferents (Fig. 4). After 7 days, both nNOS mRNA and protein expression decreased in the 21 day-old before reaching minimal levels of expression in the adult afferents. NOS activity coincided with nNOS, not eNOS, mRNA and protein expression patterns, as shown by the results of the NOS enzymatic activity assay (Fig. 5) in which NOS activity was significantly elevated in the immediate newborn period before decreasing to its lowest levels observed in the adult afferent arterioles.
Fig. 3.
Basal expression of eNOS in afferent arterioles. A.) eNOS mRNA expression as measured by real-time PCR. B.) eNOS protein expression as measured by western blot. *P<0.05, vs. NB, 7D, 21D; †P<0.05, vs. NB.
Fig. 4.
Basal expression of nNOS in afferent arterioles. A.) nNOS mRNA expression as measured by real-time PCR. B.) nNOS protein expression as measured by western blot. *P<0.05, vs. 21D, AD.
Fig. 5.
Basal NOS enzymatic activity in afferent arterioles as measured by the production of NO by quantification of 3H-L-arginine converted to 3H-L-citrulline during enzymatic activity assay. *P<0.05, vs. 21D, AD; †P<0.05, vs. AD.
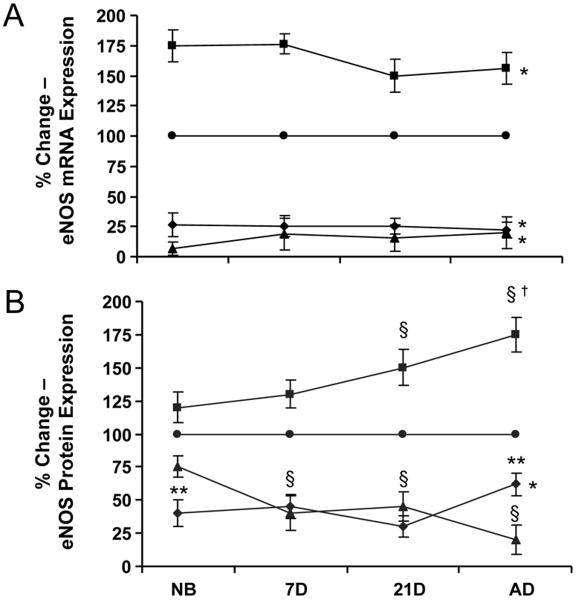
Upon ANG II treatment, afferent arterioles responded by increasing eNOS mRNA expression to 175% in the newborn and 156% in the adult afferent, an effect that was blocked by both AT1 and AT2 receptor inhibition (Fig. 6). However, while eNOS protein expression was also increased during ANG II treatment, enhanced protein expression was not significant in the newborn (expression was 120% as compared with basal protein expression) and required further vascular maturity of the afferent before ANG II induced significant upregulation of eNOS protein, as observed in the adult where expression was 175%. ANG II effects on eNOS protein expression demonstrated minimal regulation in the newborn's afferent arterioles via the AT1 receptor, but AT1 receptor modulation of ANG II regulation increased with continued maturation, while eNOS protein expression regulation via the AT2 receptor was initially elevated in the newborn before attenuation with continued maturation.
Fig. 6.
The percent change in eNOS expression in afferent arterioles in controls (●) and after treatment with ANG II (■), ANG II with candesartan (AT1X: ▲) or ANG II with PD 123319 (AT2X ◆). A.) eNOS mRNA expression as measured by real-time PCR, B.) eNOS protein expression as measured by western blot. *P<0.05, vs. control (all ages); §P<0.05, vs. control; †P<0.05, vs. NB, 7D; **P<0.05, vs. AT1X.
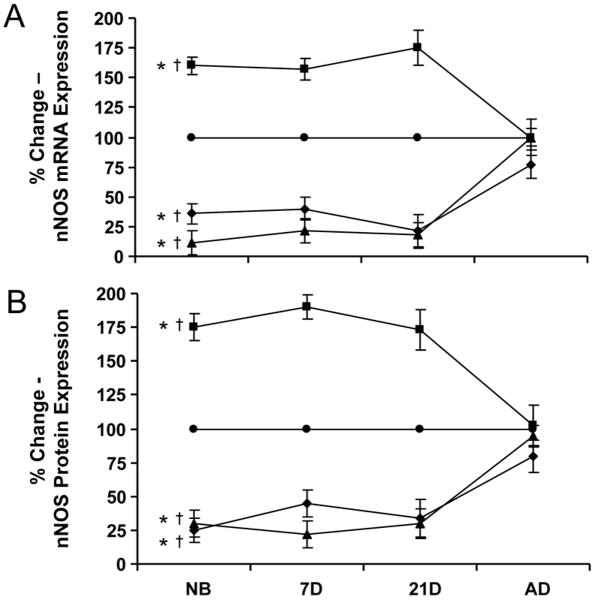
Immediately after birth, ANG II significantly upregulated both eNOS mRNA expression (160-175%) and protein (175-190%) in the afferent arterioles of the newborn, 7 day-old, and 21 day-old (Fig. 7). Blockade of either the AT1 or AT2 receptor resulted in significant blunting of both nNOS mRNA and protein in the developing afferent arterioles, suggesting their role in ANG II mediated nNOS regulation in neonate afferent arterioles. However, once arterioles were mature, ANG II had no effect on nNOS mRNA and protein expression, as observed in the afferents of the adult kidney. Furthermore, inhibition of either the AT1 or AT2 receptor in the adult did not illicit a significant reduction in nNOS mRNA or protein, suggesting ANG II does not regulate nNOS via the AT1 nor AT2 receptor of the afferent arteriole in the adult.
Fig. 7.
The percent change in nNOS expression in afferent arterioles in controls (●) and after treatment with ANG II (■), ANG II with candesartan (AT1X: ▲) or ANG II with PD 123319 (AT2X: ◆). A.) nNOS mRNA expression as measured by real-time PCR, B.) nNOS protein expression as measured by western blot. *P<0.05, vs. control (all ages except AD); †P<0.05, vs. AD (all ages).
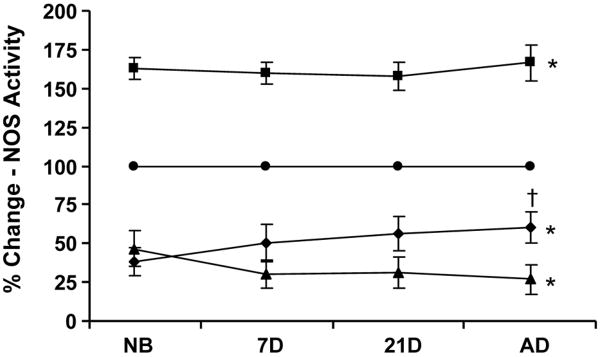
Regardless of eNOS and nNOS expression patterns, NOS enzymatic activity was enhanced equivalently (160-170% increased activity) with ANG II treatment at all ages studied (Fig. 8). After birth, modulation of ANG II regulated NOS activity by the AT1 receptor progressively increased, while AT2 receptor mediated regulation slowly diminished, with continued maturation. The treatment of afferent arterioles with either AT1 or AT2 inhibitors alone without ANG II had comparable effects on NOS isoform expression and activity as when inhibitors were introduced to arterioles in the presence of ANG II (not shown). These findings suggest nNOS may be the main contributor to NOS activity in the period immediately after birth, while eNOS assumes the role as main contributor of NOS activity in the adult, with a coordinated transition between the two enzymes during the process of maturation in the afferent arteriole.
Fig. 8.
The percent change in NOS enzymatic activity in afferent arterioles (as measured by enzymatic assay) in controls (●) and after treatment with ANG II (■), ANG II with candesartan (AT1X: ▲) or ANG II with PD 123319 (AT2X ◆). *P<0.05, vs. control (all ages); †P<0.05, vs. AT1X.
Discussion
Despite reports by Thompson and Weiner that indicate adaptations in the sensitivity of vascular smooth muscle to NO increases after birth (24), renal blood flow remains low in the newborn due to the enhanced vasoconstrictor response of the RAS (8-11). To counter-balance the elevated RAS of the newborn and to maintain adequate RBF and GFR, studies have demonstrated a crucial enhanced role of NO in the neonate's renal hemodynamic state. In 1993, Solhaug et al. demonstrated intrarenal perfusion of the NOS inhibitor L-NAME caused greater changes in RVR, RBF and GFR in the piglet than in the adult (19). Furthermore, Sener and Smith demonstrated L-NAME administration leads to an increase in RVR, a decrease in RBF and a reduction of GFR by 50% in the newborn lamb, an affect that was attenuated as the newborn lamb matured (18,25). In 1996, Ballevre et al. also demonstrated an enhanced role of NO in the neonate when NOS inhibition with L-NAME in newborn rabbits increased RVR by 31%, while decreasing RBF by 20% and GFR by 13% (17).
More recent studies have further linked ANG II to regulation of neonatal NOS expression and activity. In the neonate, nNOS has a developmental pattern similar to that of renin producing cells suggesting an interaction between the vasoconstriction of the RAS and the vasodilation effects of NOS (14,26). Solhaug et al. demonstrated pre-perfusion of an AT1 receptor inhibitor prior to NOS inhibition with L-NAME renal perfusion blunted the effects of L-NAME in the newborn, but not the adult, kidney (11). Ratliff et al. demonstrated AT1 receptor inhibition in the newborn piglet attenuated glomerular eNOS expression (27). However, information on NOS regulation is much more available in the adult. For instance, experiments by various groups have identified both nNOS and eNOS isoforms in the afferent arterioles of the adult rat (14,21,28,29). Patzak et al. observed augmented contraction of almost 25% in afferent arterioles by ANG II when NOS was inhibited (30). Patzak et al. and Thorup et al. both found ANG II administration increased NO production by 8-10% in adult afferent arterioles (31,32), while the ANG II induced increase in NO production was abolished upon blockade of the AT1 receptor (31). Furthermore, Patzak et al. demonstrated ANG II vasoconstrictor response in adult mice afferent arterioles is enhanced by 10% in eNOS knockout mice (30). Hennington et al. observed intrarenal ANG II infusion increases eNOS mRNA by 70% in adult rat kidneys (33).
Results here indicate ANG II upregulated both eNOS and nNOS mRNA and protein expression in the immature afferent arterioles. ANG II ability to upregulate eNOS protein was minimal in the newborn, possibly due to maximum cellular capacity to generate increased eNOS protein, thus increasing the need for an alternative source of NO, possibly from increased nNOS expression. Indeed, mRNA and protein expression patterns of nNOS in both whole kidney studies (15) and here utilizing isolated afferent arterioles are very similar to the developmental pattern of NOS activity, suggesting nNOS may be responsible for the enhanced NOS activity in the newborn. Both nNOS expression and NOS activity are enhanced in the neonate, in contrast to eNOS expression, which is significantly reduced in the newborn. Blockade of either AT1 or AT2 receptor attenuated eNOS and nNOS mRNA and protein expression and NOS activity in the immature afferent arterioles, a finding in agreement with the reports in the adult (31).
In contrast to findings here, a study on newborn porcine arteries by Baranov and Armstead (34) indicated ANG II primarily elicits dilation via the AT1 and not the AT2 receptor. However, Baranov and Armstead utilized pial arteries from the porcine cerebrovascular system, a vascular system different from the renal preglomerular resistance vasculature. Chappellaz and Smith (35) measured RBF changes in the renal artery during systemic injection of AT1 and AT2 receptor inhibitors in lambs and observed ANG II modulates RVR through activation of AT1, but not AT2 receptors. While Chappellaz's approach determined whole kidney RBF modulation by ANG II receptors, it failed to take in account the regulation of NOS activity by specific segments of the renal vasculature, such as the afferent arteriole, which may have functional differences as compared to the rest of the renal vasculature. Furthermore, there may be differences in vascular response with regard to species.
A contributing factor to ANG II regulation of NOS expression/activity and vasoactivity in the afferent arterioles that may account for differences observed between developmental age, vascular beds, or species may be attributed to differences in the local expression of AT1 and AT2 receptors (in addition to possible differences in receptor coupling) in the vessels. Ratliff et al. demonstrated vast differences in expression of AT1 and AT2 receptors in the afferent arterioles of the newborn and adult, and also between afferent arterioles and other arteries within the kidney (14). Ratliff et al. and others have reported AT1 receptor protein increases while AT2 receptor protein decreases in the preglomerular vasculature with continued maturation after birth (12-14). While our results here show attenuation of NOS mRNA and protein during AT1 and AT2 inhibition in the immature afferent arterioles, inhibition of either receptor in the adult had no significant effect on nNOS expression. In accord with receptor developmental expression patterns of ANG II receptors in the developing afferent arteriole, here we show NOS activity regulation by the AT1 receptor increases while AT2 receptor regulation decreases during maturation.
Although nNOS expression was similar to the developmental pattern of NOS activity, regulation of nNOS expression was slightly different than regulation of NOS activity by ANG II receptors during the immature period (NB thru 21 days) and into adulthood. Indeed, these results are unexpected but may suggest crosstalk and coordinated changes between the ANG II receptor subtypes, thus leading to compensatory mechanisms between the two receptors relating changes in NOS expressional and activity during maturation. The notion of crosstalk between the two receptors is also suggested by the expression patterns of the receptors in the afferent arteriole during maturation, as reported by Ratliff et al (14). Maternal humoral factors still present in the newborn after birth may play an important role in the developmental and coordinated changes observed in the preglomerular vasculature of the developing neonate.
ANG II may modulate NOS enzymatic activity through various pathways. AT1 receptor activation stimulates multiple mechanisms that increase intracellular calcium concentration including the opening of cell membrane calcium channels, activation of phospholipase C and IP3 formation, release of calcium from internal stores, and activation of protein kinase C (PKC). Calcium forms a complex with calmodulin and binds to NOS enzymes thus displacing inhibitory mechanisms on both eNOS and nNOS enzymes rendering the enzymes’ catalytic activity functional. ANG II activation of AT1 and AT2 receptors also results in a cascade of events that include the phosphorylation and activation of various kinases including Akt, p38 MAPK, AMPK, cAMP-dependent protein kinase (PKA), MAPK kinase, and other MAPKs, all of which lead to increased phosphorylation of NOS and increased NOS catalytic activity (36-38).
ANG II may influence NOS expression through various pathways. The nNOS gene contains potential binding sites for transcription factors such as AP-2, transcriptional enhancer factor-a/M-CAT binding factor, CREB, ATF, Ets, NF-1 and NF-κB (39), while the eNOS promoter contains consensus sequences for the binding of transcription factors AP-1, AP-2, NF-1, NF-κB, and CREB (39). Stimulation of ANG II receptors activates various MAPKs including ERK1/2 and p38 MAPK, which leads to phosphorylation of AP-1 and induction of c-fos and c-jun, and activation of CREB leading to increased binding of transcription factors to NOS promoter sequences causing an increase in NOS transcription.
Based on results here, we conclude eNOS and nNOS expression and NOS functional activity in the immature afferent arteriole is regulated by ANG II through the AT1 and AT2 receptors. Furthermore, developmental patterns of NOS expression and activity suggest nNOS is the crucial enhanced isoform that provides counter-balancing NO vasodilation thus opposing augmented RAS vasoactivity characteristic of newborn renal hemodynamics.
Acknowledgments
Financial support: This work was supported by NIH grant 2R01HL52765-04A2 [M.J.S.].
Abbreviations
- ANG II
angiotensin II
- RAS
renin-angiotensin system
- AT1X
AT1 inhibition
- AT2X
AT2 inhibition
- RBF
renal blood flow
- RVR
renal vascular resistance
- NB
newborn
- 7D
7 day-old
- 21D
21 day-old
- AD
adult
Footnotes
Publisher's Disclaimer: Pediatric Research Articles Ahead of Print contains articles in unedited manuscript form that have been peer-reviewed and accepted for publication. As a service to our readers, we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting and review of the resulting proof before it is published in its final definitive form. Please note that during the production process errors may be discovered, which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gruskin AB, Edelman CM, Jr, Yuan S. Maturational changes in renal blood flow in piglets. Pediatr Res. 1970;4:7–13. doi: 10.1203/00006450-197001000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Kleinman LI, Lubbe RJ. Factors affecting the maturation of glomerular filtration rate and renal plasma flow in the newborn dog. J Physiol. 1972;223:395–409. doi: 10.1113/jphysiol.1972.sp009854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jose PA, Logan AG, Slotkoff LM, Lilienfield LS, Calcagno PL, Eisner GM. Intrarenal blood flow distribution in canine puppies. Pediatr Res. 1971;5:335–344. doi: 10.1203/00006450-197108000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Evan AP, Jr, Stoeckel JA, Loemker V, Baker JT. Development of the intrarenal vascular system of the puppy kidney. Anat Rec. 1979;194:187–199. doi: 10.1002/ar.1091940202. [DOI] [PubMed] [Google Scholar]
- 5.Spitzer A, Edelmann CM., Jr Maturational changes in pressure gradients for glomerular filtration. Am J Physiol. 1971;221:1431–1435. doi: 10.1152/ajplegacy.1971.221.5.1431. [DOI] [PubMed] [Google Scholar]
- 6.Aperia A, Broberger O, Herin P, Joelsson I. Renal hemodynamics in the perinatal period, A study in lambs. Acta Physiol Scand. 1977;99:261–269. doi: 10.1111/j.1748-1716.1977.tb10379.x. [DOI] [PubMed] [Google Scholar]
- 7.Ito S, Johnson CS, Carretero OA. Modulation of angiotensin II-induced vasoconstriction by endothelium-derived relaxing factor in the isolated microperfused rabbit afferent arteriole. J Clin Invest. 1991;87:1656–1663. doi: 10.1172/JCI115181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osborn JL, Hook JB, Bailie MD. Regulation of plasma renin in developing piglets. Dev Pharmacol Ther. 1980;1:217–228. [PubMed] [Google Scholar]
- 9.Pelayo JC, Eisner GM, Jose PA. The ontogeny of the renin-angiotensin system. Clin Perinatol. 1981;8:347–359. [PubMed] [Google Scholar]
- 10.Wallace KB. Postnatal development of the renin-angiotensin system in rats. Am J Physiol. 1980;238:R432–R437. doi: 10.1152/ajpregu.1980.238.5.R432. [DOI] [PubMed] [Google Scholar]
- 11.Solhaug MJ, Wallace MR, Granger JP. Nitric oxide and angiotensin II regulation of renal hemodynamics in the developing piglet. Pediatr Res. 1996;39:527–533. doi: 10.1203/00006450-199603000-00023. [DOI] [PubMed] [Google Scholar]
- 12.Gomez RA, Lynch KR, Chevalier RL, Wilfong N, Everett A, Carey RM, Peach MJ. Renin and angiotensinogen gene expression in maturing rat kidney. Am J Physiol. 1988;254:F582–F587. doi: 10.1152/ajprenal.1988.254.4.F582. [DOI] [PubMed] [Google Scholar]
- 13.Ciuffo GM, Viswanathan M, Seltzer AM, Tsutsumi K, Saavedra JM. Glomerular angiotensin II receptor subtypes during development. Am J Physiol. 1993;265:F264–F271. doi: 10.1152/ajprenal.1993.265.2.F264. [DOI] [PubMed] [Google Scholar]
- 14.Ratliff B, Rodebaugh J, Sekulic M, Dong KW, Solhaug M. Nitric oxide synthase and renin-angiotensin expression and NOS function in the postnatal renal resistance vasculature. Pediatr Nephrol. 2009;24:355–365. doi: 10.1007/s00467-008-0977-9. [DOI] [PubMed] [Google Scholar]
- 15.Solhaug MJ, Dong XQ, Adelman RD, Dong KW. Ontogeny of neuronal nitric oxide synthase, NOS I, in the developing porcine kidney. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1453–R1459. doi: 10.1152/ajpregu.2000.278.6.R1453. [DOI] [PubMed] [Google Scholar]
- 16.Solhaug MJ, Kullaprawithaya U, Dong XQ, Dong KW. Expression of endothelial nitric oxide synthase in the postnatal developing porcine kidney. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1269–R1275. doi: 10.1152/ajpregu.2001.280.5.R1269. [DOI] [PubMed] [Google Scholar]
- 17.Ballevre L, Thonney M, Guignard JP. Nitric oxide modulates glomerular filtration and renal blood flow of the newborn rabbit. Biol Neonate. 1996;69:389–398. doi: 10.1159/000244336. [DOI] [PubMed] [Google Scholar]
- 18.Sener A, Smith FG. Glomerular and tubular responses to N(G)-nitro-L-arginine methyl ester are age dependent in conscious lambs. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1512–R1520. doi: 10.1152/ajpregu.00628.2001. [DOI] [PubMed] [Google Scholar]
- 19.Solhaug MJ, Wallace MR, Granger JP. Endothelium-derived nitric oxide regulates renal hemodynamics in the developing piglet. Pediatr Res. 1993;34:750–754. doi: 10.1203/00006450-199312000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Torok J, Gerova M. Vascular responses after long-term inhibition of nitric oxide synthesis in newborn dogs. Physiol Res. 1996;45:323–328. [PubMed] [Google Scholar]
- 21.Mattson DL, Wu F. Nitric oxide synthase activity and isoforms in rat renal vasculature. Hypertension. 2000;35:337–341. doi: 10.1161/01.hyp.35.1.337. [DOI] [PubMed] [Google Scholar]
- 22.Pelayo JC, Mobilia M, Tjio S, Singh R, Nakamoto JM, Van Dop C. A method for isolation of rat renal microvessels and mRNA localization. Am J Physiol. 1994;267:F497–F503. doi: 10.1152/ajprenal.1994.267.3.F497. [DOI] [PubMed] [Google Scholar]
- 23.Xiao S, Erdely A, Wagner L, Baylis C. Uremic levels of BUN do not cause nitric oxide deficiency in rats with normal renal function. Am J Physiol Renal Physiol. 2001;280:F996–F1000. doi: 10.1152/ajprenal.2001.280.6.F996. [DOI] [PubMed] [Google Scholar]
- 24.Thompson LP, Weiner CP. Acetylcholine relaxation of renal artery and nitric oxide synthase activity of renal cortex increase with fetal and postnatal age. Pediatr Res. 1996;40:192–197. doi: 10.1203/00006450-199608000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Sener A, Smith FG. Renal hemodynamic effects of L-NAME during postnatal maturation in conscious lambs. Pediatr Nephrol. 2001;16:868–873. doi: 10.1007/s004670100672. [DOI] [PubMed] [Google Scholar]
- 26.Fischer E, Schnermann J, Briggs JP, Kriz W, Ronco PM, Bachmann S. Ontogeny of NO synthase and renin in juxtaglomerular apparatus of rat kidneys. Am J Physiol. 1995;268:F1164–F1176. doi: 10.1152/ajprenal.1995.268.6.F1164. [DOI] [PubMed] [Google Scholar]
- 27.Ratliff B, Rodebaugh J, Sekulic M, Solhaug M. Glomerular eNOS expression during postnatal maturation and AT1 receptor inhibition. Pediatr Nephrol. 2007;22:1135–1142. doi: 10.1007/s00467-007-0489-z. [DOI] [PubMed] [Google Scholar]
- 28.Bachmann S, Bosse HM, Mundel P. Topography of nitric oxide synthesis by localizing constitutive NO synthases in mammalian kidney. Am J Physiol. 1995;268:F885–F898. doi: 10.1152/ajprenal.1995.268.5.F885. [DOI] [PubMed] [Google Scholar]
- 29.Terada Y, Tomita K, Nonoguchi H, Marumo F. Polymerase chain reaction localization of constitutive nitric oxide synthase and soluble guanylate cyclase messenger RNAs in microdissected rat nephron segments. J Clin Invest. 1992;90:659–665. doi: 10.1172/JCI115908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patzak A, Mrowka R, Storch E, Hocher B, Persson PB. Interaction of angiotensin II and nitric oxide in isolated perfused afferent arterioles of mice. J Am Soc Nephrol. 2001;12:1122–1127. doi: 10.1681/ASN.V1261122. [DOI] [PubMed] [Google Scholar]
- 31.Patzak A, Lai EY, Mrowka R, Steege A, Persson PB, Persson AE. AT1 receptors mediate angiotensin II-induced release of nitric oxide in afferent arterioles. Kidney Int. 2004;66:1949–1958. doi: 10.1111/j.1523-1755.2004.00981.x. [DOI] [PubMed] [Google Scholar]
- 32.Thorup C, Kornfield M, Goligorsky MS, Moore LC. AT1 receptor inhibition blunts angiotensin II stimulated nitric oxide release in renal arteries. J Am Soc Nephrol. 1999;10:S220–S224. [PubMed] [Google Scholar]
- 33.Hennington BS, Zhang H, Miller MT. Angiotensin II stimulates synthesis of endothelial nitric oxide synthase. Hypertension. 1998;31:283–288. doi: 10.1161/01.hyp.31.1.283. [DOI] [PubMed] [Google Scholar]
- 34.Baranov D, Armstead WM. Nitric oxide contributes to AT2 but not AT1 angiotensin II receptor-mediated vasodilation of porcine pial arteries and arterioles. Eur J Pharmacol. 2005;525:112–116. doi: 10.1016/j.ejphar.2005.06.052. [DOI] [PubMed] [Google Scholar]
- 35.Chappellaz ML, Smith FG. Systemic and renal hemodynamic effects of the AT1 receptor antagonist, ZD 7155, and the AT2 receptor antagonist, PD 123319, in conscious lambs. Pflugers Arch. 2007;453:477–486. doi: 10.1007/s00424-006-0148-4. [DOI] [PubMed] [Google Scholar]
- 36.Michell BJ, Chen Z, Tiganis T, Stapleton D, Katsis F, Power DA, Sim AT, Kemp BE. Coordinated control of endothelial nitric-oxide synthase phosphorylation by protein kinase C and the cAMP-dependent protein kinase. J Biol Chem. 2001;276:17625–17628. doi: 10.1074/jbc.C100122200. [DOI] [PubMed] [Google Scholar]
- 37.Jiang JG, Chen RJ, Xiao B, Wang JN, Wang Y, Cowart LA, Xiao X, Wang DW, Xia Y. Regulation of endothelial nitric oxide synthase activity through phosphorylation in response to epoxyeicosatrienoic acids. Prostaglandins Other Lipid Mediat. 2007;82:162–174. doi: 10.1016/j.prostaglandins.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 38.Gorin Y, Kim NH, Feliers D, Bhandari B, Choudhury GG, Abboud HE. Angiotensin II activates Akt/protein kinase B by an arachidonic acid/redox-dependent pathway and independent of phosphoinositide 3-kinase. FASEB J. 2001;15:1909–1920. doi: 10.1096/fj..01-0165com. [DOI] [PubMed] [Google Scholar]
- 39.Forstermann U, Boissel JP, Kleinert H. Expressional control of the constitutive isoforms of nitric oxide synthase (NOS I and NOS III) FASEB J. 1998;12:773–790. [PubMed] [Google Scholar]