Abstract
Antiviral therapies are urgently needed to control emerging flaviviruses such as dengue, West Nile, and yellow fever. Ribavirin (RBV) has shown activity against flaviviruses in cultured cells, but efficacy in animal models has generally been poor. In a preliminary screen of novel, synthetic 1-β-D-ribofuranosyl-azole analogs, two compounds, 1-β-D-ribofuranosyl-3-ethynyl-[1,2,4]triazole (ETAR) and 1-β-D-ribofuranosyl-4-ethynyl[1,3]imidazole (IM18), significantly reduced replication of dengue virus serotype 2 (DENV-2) in cultured Vero cells. In the current study we demonstrated that the effective concentration 50 (EC50) of ETAR for DENV-2 is substantially lower than both IM18 and RBV. Moreover ETAR reduced the replication of five additional flaviviruses, including DENV serotypes 1, 3 and 4, Langat virus and Modoc virus, ≥ 1000-fold relative to untreated controls. Addition of exogenous guanosine to DENV-2 infected cells negated the antiviral effects of both RBV and ETAR, indicating that GTP depletion is a major mechanism of action for both drugs. ETAR represents a promising drug candidate for treatment of flavivirus infections.
Keywords: dengue virus, ribavirin, antiviral, nucleoside analog, ETAR, flavivirus
1. Introduction
The genus Flavivirus (family Flaviviridae) consists of more than 80 species of single-stranded, positive-sense RNA viruses (Cook and Holmes, 2006), including a number of globally significant emerging pathogens such as dengue virus (DENV) (Kyle and Harris, 2008), West Nile virus (WNV) (Kramer et al., 2008), and tick-borne encephalitis virus (Randolph, 2009). Effective antiviral therapies, currently unavailable for any flavivirus, are urgently needed to ameliorate the disease burden imposed by flaviviruses (Ghosh and Basu, 2008; Sampath and Padmanabhan, 2009; Stein and Shi, 2008). Ribavirin (RBV) has shown activity against all flaviviruses tested in a broad array of cell types in vitro but efficacy in vivo has generally been poor (Monath, 2008; Sampath and Padmanabhan, 2009). Additionally, RBV can be toxic in vivo (Bodenheimer et al., 1997; Russmann et al., 2006). A compound that exhibited a lower effective dose and toxicity than RBV while retaining its broad spectrum of activity would be particularly desirable as a candidate flavivirus therapy (Sampath and Padmanabhan, 2009).
We have previously synthesized a panel of 21 novel nucleoside analogs, some based on the structure of RBV (Chung et al., 2008; Kumarapperuma et al., 2007). One of these compounds, 1-β-D-ribofuranosyl-3-ethynyl-[1,2,4]triazole (ETAR) inhibited replication of Hantaan and Andes virus with effective concentration 50 (EC50) values of 10 and 4.4 μM, respectively (Chung et al., 2008). A previous screen at 50 μM showed that two of the 21 compounds, ETAR and 1-β-D-ribofuranosyl-4-ethynyl[1,3]imidazole (IM-18), inhibited DENV serotype 2 (DENV-2) replication in Vero cells by tenfold. Both compounds possess an ethynyl group and isostructural relationship to RBV through replacement of the 3-carboxamide group of the parent scaffold (Figure 1A).
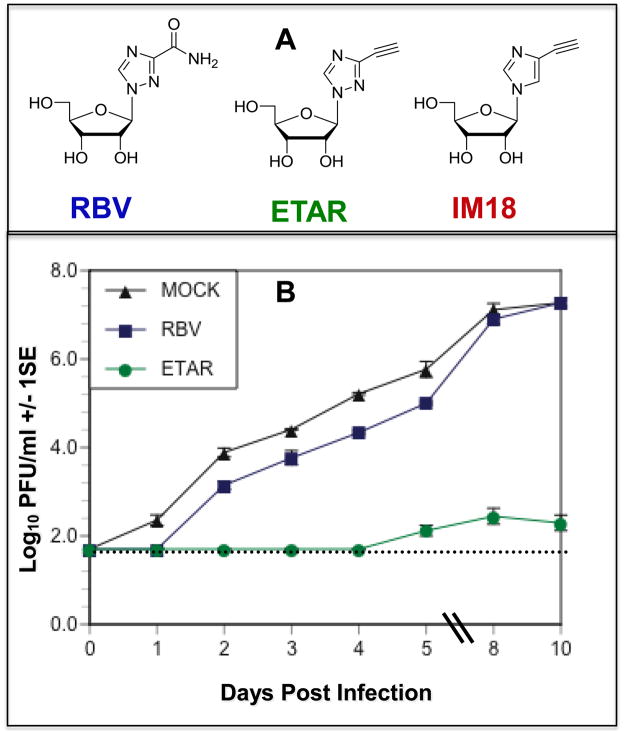
Figure 1.
A) Structure of RBV, ETAR and IM-18. B) Replication kinetics of DENV-2 in Vero cells infected at MOI 0.1 and treated with 50 μM ETAR, RBV, or media (mock) 2 hours post-infection. Dashed line indicates limit of detection of the assay.
Efficacy of both compounds was compared to RBV to assess the relative effect of the alkyne-substituents and the effect of replacing a nitrogen atom with CH at the 2-position of the heterocycle. Each compound was diluted in water to create a 10 mM stock and subsequently diluted in cell culture media. Vero cells were grown to confluency in 24 well plates as previously described (Hanley et al., 2003), media was removed, DENV-2 was added at a multiplicity of infection (MOI) of 0.1 in 100 μL of media and allowed to adsorb for 2 hours, and then 900 μL of each compound was added to quadruplicate wells in serial twofold dilutions, giving final concentrations ranging from 400 μM to 1.6 μM. Control cells were infected and mock-treated with media. Cells were incubated for 5 days and supernatants were harvested and titered via serial dilution followed by immunostaining as previously described (Hanley et al., 2003). The EC50 and EC90 of each compound was determined using a 4 parameter, nonlinear regression of dose response inhibition by plotting log (inhibitor(concentration)) vs. viral titer (variable slope) using GraphPad Prism (GraphPad Software, San Diego, CA). The EC50 of ETAR was 9.5 μM, an order of magnitude lower that that of RBV, which was 73.2 μM, a typical value for the efficacy of RBV against DENV infecting this cell type (Buckwold et al., 2007; Crance et al., 2003; Day et al., 2005; Huggins et al., 1984; Julander et al., 2007; Kirsi et al., 1983; Leyssen et al., 2000; Van Aerschot et al., 2003). The EC50 of IM-18, 106.1 μM, was similar to that of RBV and thus IM-18 was not characterized further. The EC90 values were 176.9, 259.7, and 402.9 μM for ETAR, RBV, and IM18 respectively.
To measure the effect of ETAR on virus replication kinetics, replicate wells of Vero cell monolayers were infected with DENV-2 and treated with ETAR or RBV or mock-treated with media as described above, and cell supernatants were harvested from quadruplicate wells from each treatment on days 0–5 and 8 post infection. Treatment with 50 μM ETAR delayed the onset of detectable replication by four days and suppressed titer at day 5 post-infection 100,000-fold relative to the control (Figure 1B). In contrast, treatment with 50 μM RBV delayed the onset of detectable virus replication by only one day and no difference in virus titer between the RBV treatment and the control treatment was evident by day 5 post-infection (Figure 1B). The data in Figure 1B are representative of multiple, similar experiments that we have conducted, in which treatment with 50 μM ETAR delayed viral replication by 2 to 4 days and suppressed peak titer by 1,000 to 100,000 fold. To determine the breadth of efficacy of ETAR against flaviviruses, Vero cell monolayers were infected as described above with DENV-1, 2, 3 or 4, Langat virus (LGTV) or Modoc virus (MODV) at MOI 0.1 in triplicate and treated with 50 μM ETAR or RBV or mock-treated with media. ETAR inhibited the replication of all six viruses by ≥ 1000-fold (Figure 2) relative to mock-treated cells while RBV caused only about a five-fold suppression. In this experiment ETAR suppressed replication of DENV-2 by 10,000 fold, slightly less than its effect in Figure 1B. In many similar experiments we have conducted, the minimum suppression of any of the designated flaviviruses treated with 50 μM ETAR was 100-fold. In combination with the finding that ETAR inhibits replication of bunyaviruses (Chung et al., 2008), these data suggest that ETAR may possess the same broad spectrum of activity as RBV.
Figure 2.
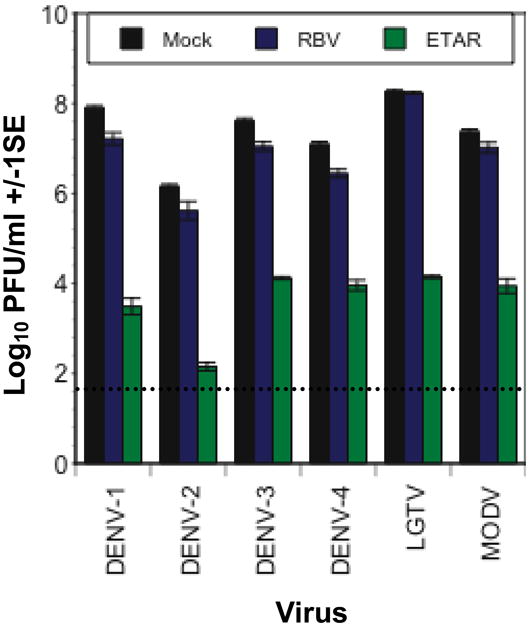
Efficacy of 50 μM ETAR against six flaviviruses in Vero cells. Dashed line indicates limit of detection of the assay.
To assess toxicity of ETAR, Vero cells were propagated to confluency in 96-well tissue culture treated plates and media was removed. RBV or ETAR was added to wells in triplicate to give 2 fold increasing concentrations from 25 to 1000 μM in a total volume of 100 μl. Mock-treated cells received 100 μL of media. Plates were incubated as described above for 5 days, after which 10 μL of resazurin (In Vitro Toxicology Assay Kit, Sigma Aldrich, St. Louis, MO) was added to all wells. Plates were incubated 2 hours and absorbance was read on a plate reader (TiterTek, Huntsville, AL USA) at 600 nm. Cytotoxicity for Vero cells was not detected at 1000 μM, the highest concentration used, for ETAR or RBV. Thus the cytotoxic concentration 50 (CC50) of ETAR greatly exceeded its EC50. Chung et al. (Chung et al., 2008) also found that ETAR was not toxic to Vero cells at concentrations below 880 μM. However the CC50 of ETAR for proliferating CEM cells was 7 μM. ETAR caused no clinical symptoms in suckling mice treated with 12.5 mg/kg, but treatment with 50 mg/kg caused 20% mortality (Chung et al., 2008).
Five different mechanisms for the antiviral action of RBV have been proposed (Graci and Cameron, 2006): (1) inhibition of the enzyme inosine monophosphate dehydrogenase (IMPDH), (2) inhibition of viral RNA capping, (3) inhibition of the viral RNA-dependent RNA polymerase (RdRp), (4) mutagenesis, leading to error catastrophe, and (5) immuno-modulation promoting a Th1 type immune response. Although RBV has been shown to cause error catastrophe and inhibition of methyltransferase in WNV (Day et al., 2005) and DENV-1(Benarroch et al., 2004), respectively, the major mode of action of RBV against most flaviviruses appears to be inhibition of IMPDH, as demonstrated by the ability of supplementary guanosine to reverse the antiviral effect of RBV (Leyssen et al., 2005; Takhampunya et al., 2006)
To assess the importance of IMPDH inhibition for ETAR activity, Vero cell monolayers were infected with DENV-2 as described above. Triplicate wells were treated with either ETAR or RBV at final concentration of 12.5 μM or 200 μM, respectively, as described above, to reduce viral titer by at least 50% relative to mock treated virus controls. Triplicate wells were also treated concurrently with one of the two drugs at the specified concentrations along with either 12.5 μM guanosine (Sigma), adenosine (Sigma) or cytidine (Sigma). Additionally, triplicate wells of DENV-2 infected cells were treated with each nucleoside alone at 12.5 μM, or mock-treated with media. Cell supernatants were harvested 5 days post infection, stored and titered as described above. Treatment with RBV reduced DENV-2 replication by approximately 100-fold; addition of guanosine but not adenosine or cytidine reversed this effect. Similarly, guanosine but not adenosine or cytidine reversed the 50-fold reduction in titer caused by ETAR. None of the three nucleosides affected the level of viral replication in the absence of an antiviral drug treatment.
Chung et al. (Chung et al., 2008) reported that treatment of Vero cells with 42 μM ETAR and 42 μM RBV for 4 hours resulted in reduction of GTP levels by 79% and 42%, respectively. In contrast with the findings of the current study, they also found that supplementation with guanosine had little effect on the efficacy of RBV against Hantaan virus (Sun et al., 2007) and curtailed but did not abolish the antiviral effect of ETAR (Chung et al., 2008). In combination, these data suggest that ETAR enacts both direct antiviral effects on bunyaviruses as well as indirect effects mediated by GTP depletion, while its antiviral effects on flaviviruses are wholly attributable to GTP depletion.
In conclusion, ETAR is a promising, broad-spectrum antiviral drug with substantially higher efficacy than RBV against flaviviruses and comparable toxicity in vitro. Moreover, the findings in the current study suggest that ETAR, like RBV, exerts its antiflaviviral effects via inhibition of IMPDH. The greater efficacy of ETAR in vitro offers hope that the drug may be efficacious in vivo at significantly lower concentrations than RBV, thereby mitigating its potential toxicity.
Acknowledgments
Funding was provided by an NMSU Interdisciplinary Research grant and NIH NM-INBRE 2P20RR01648-09. SG was supported by the NMSU Minority Biomedical Research Support-Research Initiative for Scientific Enhancement (MBRS-RISE) National Institute of Health Grant (GM61222). We thank Drs. Aravinda de Silva, Alexander Pletnev, Robert Tesh and Stephen Whitehead for providing viruses and other reagents.
Abbreviations
- ETAR
1-β-D-ribofuranosyl-3-ethynyl-[1,2,4]triazole
- IM18
1-β-D-ribofuranosyl-4-ethynyl[1,3]imidazole
- DENV
dengue virus
- LGTV
Langat virus
- MODV
Modoc virus
- WNV
West Nile virus
- RBV
ribavirin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson JF, Rahal JJ. Efficacy of interferon alpha-2b and ribavirin against West Nile virus in vitro. Emerg Infect Dis. 2002;8:107–108. doi: 10.3201/eid0801.010252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch D, Egloff MP, Mulard L, Guerreiro C, Romette JL, Canard B. A structural basis for the inhibition of the NS5 dengue virus mRNA 2′-O-methyltransferase domain by ribavirin 5′-triphosphate. J Biol Chem. 2004;279:35638–35643. doi: 10.1074/jbc.M400460200. [DOI] [PubMed] [Google Scholar]
- Bodenheimer HC, Jr, Lindsay KL, Davis GL, Lewis JH, Thung SN, Seeff LB. Tolerance and efficacy of oral ribavirin treatment of chronic hepatitis C: a multicenter trial. Hepatology. 1997;26:473–477. doi: 10.1002/hep.510260231. [DOI] [PubMed] [Google Scholar]
- Buckwold VE, Wei J, Huang Z, Huang C, Nalca A, Wells J, Russell J, Collins B, Ptak R, Lang W, Scribner C, Blanchett D, Alessi T, Langecker P. Antiviral activity of CHO-SS cell-derived human omega interferon and other human interferons against HCV RNA replicons and related viruses. Antiviral Res. 2007;73:118–125. doi: 10.1016/j.antiviral.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Chung DH, Kumarapperuma SC, Sun Y, Li Q, Chu YK, Arterburn JB, Parker WB, Smith J, Spik K, Ramanathan HN, Schmaljohn CS, Jonsson CB. Synthesis of 1-beta-D-ribofuranosyl-3-ethynyl-[1,2,4]triazole and its in vitro and in vivo efficacy against Hantavirus. Antiviral Res. 2008;79:19–27. doi: 10.1016/j.antiviral.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook S, Holmes EC. A multigene analysis of the phylogenetic relationships among the flaviviruses (Family: Flaviviridae) and the evolution of vector transmission. Arch Virol. 2006;151:309–325. doi: 10.1007/s00705-005-0626-6. [DOI] [PubMed] [Google Scholar]
- Crance JM, Scaramozzino N, Jouan A, Garin D. Interferon, ribavirin, 6-azauridine and glycyrrhizin: antiviral compounds active against pathogenic flaviviruses. Antiviral Res. 2003;58:73–79. doi: 10.1016/s0166-3542(02)00185-7. [DOI] [PubMed] [Google Scholar]
- Day CW, Smee DF, Julander JG, Yamshchikov VF, Sidwell RW, Morrey JD. Error-prone replication of West Nile virus caused by ribavirin. Antiviral Res. 2005;67:38–45. doi: 10.1016/j.antiviral.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Ghosh D, Basu A. Present perspectives on flaviviral chemotherapy. Drug Discov Today. 2008;13:619–624. doi: 10.1016/j.drudis.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Graci JD, Cameron CE. Mechanisms of action of ribavirin against distinct viruses. Rev Med Virol. 2006;16:37–48. doi: 10.1002/rmv.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley KA, Manlucu LR, Gilmore LE, Blaney JE, Jr, Hanson CT, Murphy BR, Whitehead SS. A trade-off in replication in mosquito versus mammalian systems conferred by a point mutation in the NS4B protein of dengue virus type 4. Virology. 2003;312:222–232. doi: 10.1016/s0042-6822(03)00197-1. [DOI] [PubMed] [Google Scholar]
- Huggins JW, Robins RK, Canonico PG. Synergistic antiviral effects of ribavirin and the C-nucleoside analogs tiazofurin and selenazofurin against togaviruses, bunyaviruses, and arenaviruses. Antimicrob Agents Chemother. 1984;26:476–480. doi: 10.1128/aac.26.4.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julander JG, Morrey JD, Blatt LM, Shafer K, Sidwell RW. Comparison of the inhibitory effects of interferon alfacon-1 and ribavirin on yellow fever virus infection in a hamster model. Antiviral Res. 2007;73:140–146. doi: 10.1016/j.antiviral.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsi JJ, North JA, McKernan PA, Murray BK, Canonico PG, Huggins JW, Srivastava PC, Robins RK. Broad-spectrum antiviral activity of 2-beta-D-ribofuranosylselenazole-4-carboxamide, a new antiviral agent. Antimicrob Agents Chemother. 1983;24:353–361. doi: 10.1128/aac.24.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer LD, Styer LM, Ebel GD. A global perspective on the epidemiology of West Nile virus. Annu Rev Entomol. 2008;53:61–81. doi: 10.1146/annurev.ento.53.103106.093258. [DOI] [PubMed] [Google Scholar]
- Kumarapperuma SC, Sun Y, Jeselnik M, Chung K, Parker WB, Jonsson CB, Arterburn JB. Structural effects on the phosphorylation of 3-substituted 1-beta-D-ribofuranosyl-1,2,4-triazoles by human adenosine kinase. Bioorg Med Chem Lett. 2007;17:3203–3207. doi: 10.1016/j.bmcl.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle JL, Harris E. Global spread and persistence of dengue. Annu Rev Microbiol. 2008;62:71–92. doi: 10.1146/annurev.micro.62.081307.163005. [DOI] [PubMed] [Google Scholar]
- Leyssen P, Balzarini J, De Clercq E, Neyts J. The predominant mechanism by which ribavirin exerts its antiviral activity in vitro against flaviviruses and paramyxoviruses is mediated by inhibition of IMP dehydrogenase. J Virol. 2005;79:1943–1947. doi: 10.1128/JVI.79.3.1943-1947.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyssen P, De Clercq E, Neyts J. Perspectives for the treatment of infections with Flaviviridae. Clin Microbiol Rev. 2000;13:67–82. doi: 10.1128/cmr.13.1.67-82.2000. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monath TP. Treatment of yellow fever. Antiviral Res. 2008;78:116–124. doi: 10.1016/j.antiviral.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Randolph SE. To what extent has climate change contributed to the recent epidemiology of tick-borne diseases? Vet Parasitol. 2009 doi: 10.1016/j.vetpar.2009.09.011. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Russmann S, Grattagliano I, Portincasa P, Palmieri VO, Palasciano G. Ribavirin-induced anemia: mechanisms, risk factors and related targets for future research. Curr Med Chem. 2006;13:3351–3357. doi: 10.2174/092986706778773059. [DOI] [PubMed] [Google Scholar]
- Sampath A, Padmanabhan R. Molecular targets for flavivirus drug discovery. Antiviral Res. 2009;81:6–15. doi: 10.1016/j.antiviral.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein DA, Shi PY. Nucleic acid-based inhibition of flavivirus infections. Front Biosci. 2008;13:1385–1395. doi: 10.2741/2769. [DOI] [PubMed] [Google Scholar]
- Sun Y, Chung DH, Chu YK, Jonsson CB, Parker WB. Activity of ribavirin against Hantaan virus correlates with production of ribavirin-5′-triphosphate, not with inhibition of IMP dehydrogenase. Antimicrob Agents Chemother. 2007;51:84–88. doi: 10.1128/AAC.00790-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takhampunya R, Ubol S, Houng HS, Cameron CE, Padmanabhan R. Inhibition of dengue virus replication by mycophenolic acid and ribavirin. J Gen Virol. 2006;87:1947–1952. doi: 10.1099/vir.0.81655-0. [DOI] [PubMed] [Google Scholar]
- Van Aerschot A, Schepers G, Busson R, Rozenski J, Neyts J, De Clercq E, Herdewijn P. Ribavirin derivatives with a hexitol moiety: synthesis and antiviral evaluation. Antivir Chem Chemother. 2003;14:23–30. doi: 10.1177/095632020301400102. [DOI] [PubMed] [Google Scholar]