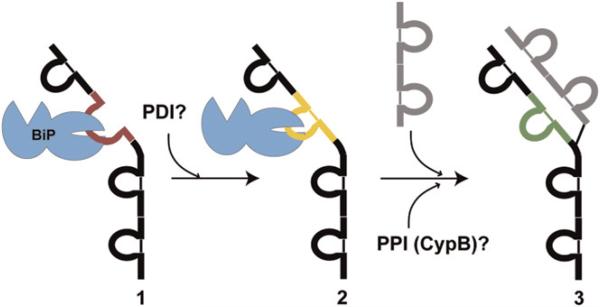
Figure 1. The Sequence of Events in Cellular Folding and Assembly of IgG Antibodies.
(1) The unfolded CH1 domain (red) of a newly synthesized IgG heavy chain is bound by BiP. (2) The intramolecular disulfide bond in the CH1 domain (yellow) forms, possibly while the domain is bound to BiP, or upon its release, and most likely catalyzed by a PDI family member. (3) The CH1 domain folds to its native structure (green) upon interaction with the complementary CL domain within its partner light chain (gray structure). Peptidyl-proline isomerization must accompany native structure formation and most likely is catalyzed by an ER PPIase such as cyclophilin B. In this cartoon, we show only one of the two heavy and two light chains in the final assembled antibody.