Figure 1.
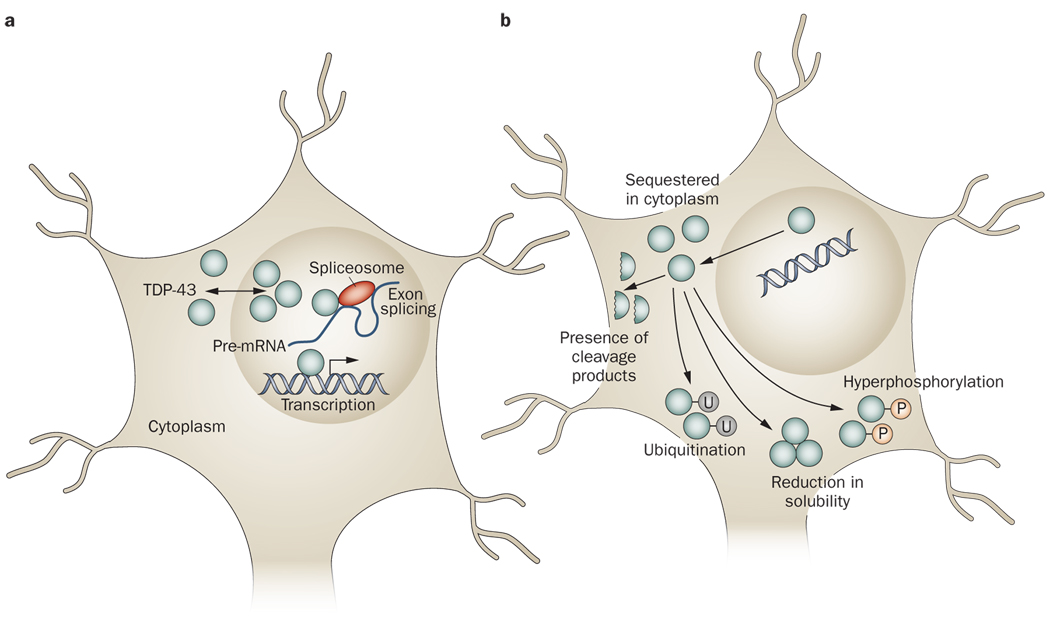
Physiological and pathophysiological TDP-43. a | Under physiological conditions, TDP-43 primarily resides in the nucleus, although the protein has the capacity to shuttle between the nucleus and the cytoplasm. TDP-43 has been reported to have roles in regulating gene expression at the transcriptional level, regulating gene splicing, and stabilizing mRNA. b | Under pathophysiological conditions, TDP-43 is cleared from the nuclear compartment and accumulates in the cytoplasm. Pathological TDP-43 has been demonstrated to be hyperphosphorylated and/or ubiquitinated. Furthermore, small carboxy-terminal fragments of TDP-43 can be found in the disease state. Pathological TDP-43 is also less soluble than TDP-43 under physiological conditions, possibly making the former more prone to aggregation. The changes that occur in the nature of TDP-43 under pathophysiological conditions might result in various loss-of-function or toxic-gain-of-function mechanisms of pathogenesis. Abbreviation: mRNA, messenger RNA; P, phosphate group; TDP-43, TAR DNA-binding protein 43; U, ubiquitin group.