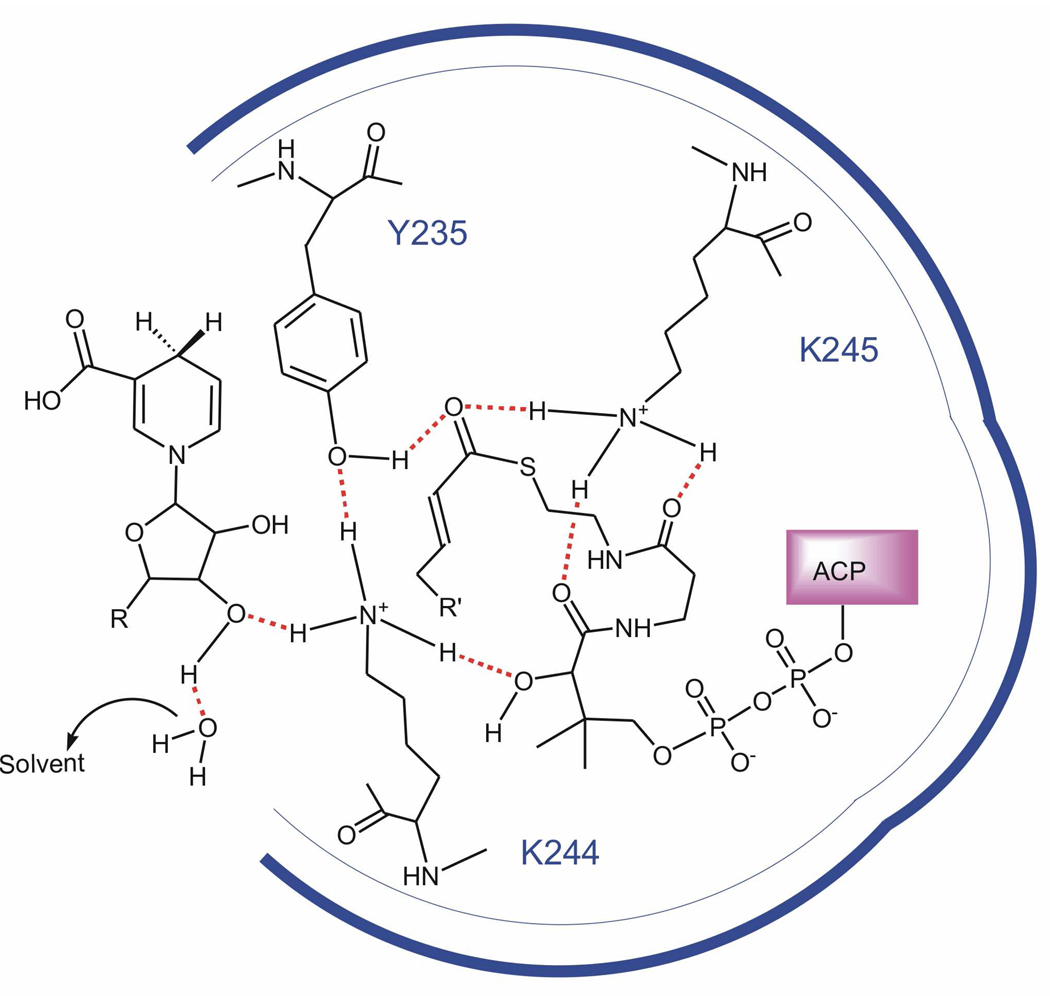
FIGURE 4.
Proposed hydrogen bonding network between the three active site residues (Y235, K244 and K245), cofactor and the enoyl–ACP substrate. Y235 stabilizes the transition state of the enoyl-ACP substrate through a hydrogen bond with the carbonyl group while K244 interacts directly with Y235 as proposed in the SDR dehydrogenases. K244 is also shown interacting with the NADH ribose hydroxyl and the secondary hydroxyl group in the ACP pantetheine, in order to account for the role of this residue in binding cofactor and substrate. K245 is shown interacting with the thioester carbonyl group, to account for the effect of mutating this residue on kcat, and also with the ACP pantetheine.