Abstract
We describe a unique spontaneous mouse model of autoimmunity, which occurs on a nonautoimmune-prone SWR genetic background. In this model, SWR mice carry an IghV partial transgene encoding only the heavy chain variable domain of an antibody directed against chromatin. Autoimmune disease in partial transgene mice was manifested by some of the features of systemic lupus erythematosus (SLE), including the presence of serum anti-nuclear antibodies, splenomegaly, skin lesions and a moderate degree of kidney pathology, in various combinations among individuals. Autoimmunity was observed in three independent transgenic lines, but not in three control lines carrying a nearly identical partial transgene, in which a VHCDR3 codon for Arg was replaced by one for Ser in order to ablate chromatin-reactivity. Various features of disease were often but not always accompanied by anti-chromatin antibodies. Unexpectedly, the anti-chromatin antibodies detected in seropositive animals were not encoded by the partial transgene. These observations strongly implicate a role for the transgene product in disease initiation but not necessarily for end-state pathology, and they raise the possibility that autoreactive B cells may play a previously unappreciated role in initiating the development of systemic autoimmunity.
Keywords: Autoantibodies, Autoimmunity, Systemic Lupus Erythematosus, Partial Transgene
Introduction
SLE is a systemic autoimmune disease or group of related diseases with numerous manifestations involving multiple organ systems that include the kidney, the skin and sometimes the lung (1). Much of the pathology is thought to be induced by immune complexes between autoantibodies and ubiquitous nuclear antigens. And many of the autoantibodies are derived from B cells that appear to be driven in a T cell-dependent immune response, indicating breaches in both T- and B-lymphocyte self-tolerance (2-5).
Although numerous self-antigens targeted by SLE autoantibodies have been defined, the antigenic specificity of T cell help for autoreactive B cells has proved to be elusive and controversial (6-10). Nevertheless, T cell help for autoreactive B cells seems to be a major rate-limiting step in disease. This can be inferred from studies in which anti-nuclear antibodies are induced when an arbitrary avenue of T cell help to anti-nuclear B cells is provided. Many investigators have reported this, even in mice that are not genetically predisposed to develop systemic autoimmunity (10-16). As such, it is tempting to conclude that the kinetics of autoantibody development in SLE is limited primarily or exclusively by the availability of autoreactive T helper cells.
On the other hand, it has proved more difficult to directly test whether the development of anti-nuclear B antibodies in SLE is also kinetically limited by the frequency of nuclear antigen-specific B cells in the repertoire. While it is clear that the B cell repertoire contains a substantial frequency of low-avidity polyreactive members, it is unclear whether these are the actual antecedents of the T cell-dependent clones that eventually emerge in SLE and secrete anti-nuclear antibodies of high avidity (17-21). Studies in mice that carry Ig transgenes, encoding anti-nuclear antibodies have not unambiguously resolved this issue because the transgenic autoreactive B cells are subjected to self-tolerance mechanisms (21-23). This is probably why anti-nuclear antibody levels are relatively modest and often undetectable in mice that carry Ig transgenes derived from hybridomas producing anti-nuclear antibodies (24-29). In addition, it appears that many of the autoreactive B cell clones that ultimately escape in such transgenic mice, edit their receptors by RAG-mediated recombination (24, 30-32). These observations suggest that immature bone marrow B cells that are “born” with an anti-nuclear B cell receptor (BCR) are not necessarily the immediate and unedited precursors of the high-avidity autoreactive clones that emerge in SLE.
The problem of not being able to increase the frequency of BCR-defined immunocompetent precursors to autoreactive clones is compounded by a lack of knowledge regarding exactly which subpopulation(s) of B cells are eligible, or at what stage of development they are eligible, for recruitment into an autoimmune response. A bone marrow B cell that is “born” with an autoreactive receptor must traverse all developmental stages and associated self-tolerance checkpoints to participate in autoimmunity. This could partly explain why mice that carry anti-nuclear Ig transgenes generally produce no or limited quantities of such autoantibodies. On the other hand, a B cell that acquires an autoreactive BCR via genetic means at a late stage in development would have to escape fewer tolerance checkpoints before differentiating into an antibody-secreting cell. Therefore, despite the relatively modest effects of Ig transgenes on autoantibody development, it is possible that a rate limiting step in autoimmunity is the presence of B cells with autoreactive receptors in a specific niche and at a particular moment. On the basis of this consideration, we asked what would happen if a B cell could recombine and express a VH/D/JH gene for an anti-nuclear antibody independently of the RAG-mediated mechanism that normally restricts this to pro- and pre- B cells.
To this end, we created mice carrying an IghV partial transgene encoding a VH/D/JH domain, derived from a hybridoma producing an antibody to a complex of histone 2A, 2B and dsDNA (H2A/H2B/dsDNA). Partial transgenes recombine into the Igh locus at a low frequency by homologous recombination in the JH intron to generate a complete functional Ig gene (33-36). Because the recombination mechanism does not require RAG enzymes, B cells that recombine and express a VH/D/JH partial transgene do not necessarily have to pass all of the developmental stages and tolerance checkpoints while expressing the transgene-encoded receptor.
We found that approximately one quarter of the partial transgene mice from 3 independent founders developed autoimmunity with some of the features of SLE. This disease occurred in mice of a nonautoimmune-prone SWR genetic background. It did not occur in 3 independent lines of SWR mice carrying a version of the partial transgene that was modified at one Arg codon previously shown to be essential for the chromatin specificity of the original monoclonal antibody (37). Unexpectedly, we could find no evidence that the transgene product was involved in end-state pathology, as might be expected of an autoantibody.
Materials and Methods
Mice
SWR/J were purchased from Jackson Laboratory. All mice were bred in our facility and used according to an IACUC approved animal protocol. All IghV partial transgene (pTg) mice were initially generated on an SWR/J background, as described previously (33). Two versions of pTg mice were developed: pTg18R and pTg104RS. The pTg18R encodes the heavy chain V domain of an antibody specific for a complex of H2A/H2B/dsDNA. The original hybridoma (SN5-18) was generated from a spontaneously autoimmune (NZB × SWR)F1 mouse (3, 8). Two somatic mutations in the VH region that had no influence on chromatin-specificity were eliminated to produce pTg18R (8). In pTg104RS, an arginine codon at position 104 in CDR3 was additionally converted to a serine codon to eliminate chromatin specificity in the corresponding antibody (37). The partial transgenes contained approximately 1 kb of DNA upstream of the leader AUG start codon and approximately 1.6 kb of downstream DNA that included the JH cluster and the intron (mu) enhancer (Figure 1A). Three founder lines of SWR mice for each construct were obtained by injecting SWR eggs with EcoRI fragments of DNA (3 kb) devoid of bacterial sequences.
FIGURE 1.
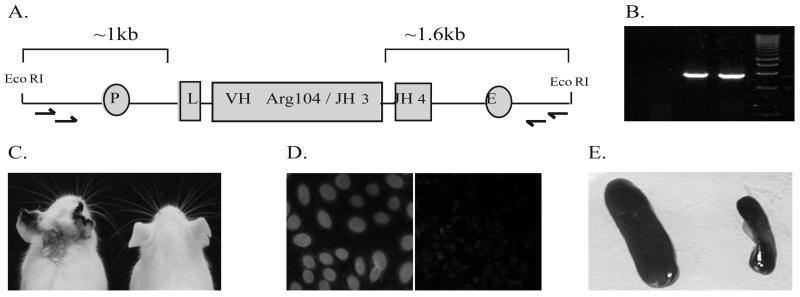
Partial transgene construct design and disease manifestations. A, Schematic illustration of pTg18R construct that was injected into fertilized SWR eggs and PCR products (B) resulting from amplification of genomic DNA with indicated primers (arrows in A), as described in Materials and Methods. Promoter (P), leader (L) and enhancer (E) positions are indicated (Figure is not to scale). Note that the constructs lack constant region exons. Example of skin lesion (C), nuclear staining of HEp-2 cells using sera (diluted 1/50) from one pTg18R animal (D, left panel) but not another (D, right panel) and splenomegaly (E).
PCR and sequencing of genomic partial transgenes
Partial transgenes were amplified from genomic DNA in a nested PCR reaction, using primers Forward 1 (5′ACTGGCAAGGATTCACAGCAA 3′) and Reverse 1 (5′GAAATGCAAATTACCCAGGTGG 3′) for the first round, Forward 2 (5′ACTCTTCCATTGCTGGTGGGATTTC 3′) and Reverse 2 (5′TTTGCTCAGCCTGGACTTTCGG 3′) for the second round (Figure 1A arrows). All the reactions were performed using a high-fidelity DNA polymerase with proof-reading function, Phusion DNA Polymerase (Finnzymes, Espoo, Finland) according to the manufacturer's specifications. The PCR products were extracted from an agarose gel following electrophoresis and sequenced to confirm that they contained intact promoter and enhancer elements.
Assessing autoimmunity in partial Tg mice
Sera of partial transgene and wild type SWR mice were screened every three weeks for anti-chromatin antibodies and for physical signs of illness. The latter included skin lesions on the back of the neck and ears, splenomegaly (postmortem) and kidney pathology with Ig and complement factor 3 (C3) depositions (postmortem). Diseased animals, or animals that appeared healthy at 12 months of age were sacrificed, and organs were collected for further analysis. B cell hybridomas were generated from splenocytes of some animals using a standard procedure (38).
Kidney histology
Kidneys were frozen in Optimal Cutting Temperature (O.C.T.) Compound (Sakura Finekek Incorporated, Torrance, CA) or fixed in 2% formalin for at least 24 hours. Ig and C3 deposits were detected by immunofluorescence (IF), using FITC goat anti-mouse IgM, IgG or C3, using a in OCT frozen samples as described (10). Formalin fixed samples were stained with hematoxilin and eosin (H&E). All samples were blindly analyzed and scored.
Anti-nuclear antibodies
HEp-2 staining was performed on NOVA-Lite HEp-2 cells (Inova Diagnostics, San Diego, CA) using sera at a dilution of 1:50 followed by FITC goat anti-mouse IgG (heavy chain-specific) (Sigma, St. Louis, MO). Anti-chromatin IgG antibodies were detected by first coating 96-well microtiter trays with mouse chromatin (10 μg/ml), followed by incubation with blocking buffer solution [2 mg/ml bovine serum albumin (BSA), 1 mg/ml gelatin, 0.02% thimerosal, and 0.05% Tween-20 (Fisher Biotech, Fair Lawn, NJ)] at 37°C for 2 hours. Mouse sera diluted in blocking buffer (1:100) was added to the trays for one hour. IgG anti-chromatin antibodies were detected with a biotinylated goat anti-mouse IgG (heavy chain-specific) antibody (Southern Biotechnology Associates, Inc., Birmingham, AL). Bound antibodies were quantified using a europium (Eu3+)-based fluorimetric immunossay as described (10), with one modification: the enhancement solution was prepared according to Keelan et al. (39). To control for nonspecific binding, serum samples were tested against BSA-coated trays and the bound radioactivity was subtracted from that of the chromatin-coated trays. Anti-chromatin antibody concentrations were calculated using the SN5-18 antibody to construct a standard curve (37). Total IgG antibodies were assayed in a similar manner, except that trays were coated with goat anti-mouse Ig (H+L) at 1 μg/ml (Southern Biotechnology Associates, Inc., Birmingham, AL). For chromatin-binding studies, of Figure 5C, IgG antibodies were purified and stripped of nuclear material as described (37).
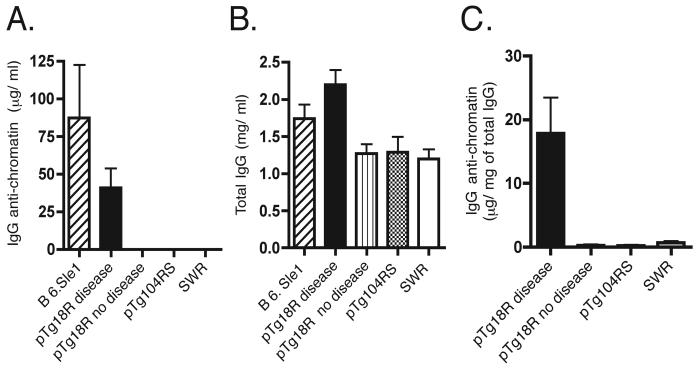
FIGURE 5.
IgG anti-chromatin as a product of clonal selection. IgG anti-chromatin (A) and total IgG (B) in pTg18R sera were quantified as described in the Materials and Methods. Asterisk indicates that counts bound to chromatin-coated trays were less than or equal to zero after subtracting counts bound to BSA-coated control trays. B6.Sle1 mice were 5 months old. C, IgG anti-chromatin normalized to total IgG. Assay was performed with purified serum IgG that was treated to remove contaminating nuclear antigens. Results are expressed as micrograms of IgG anti-chromatin per milligram of total purified IgG to control for nonspecific binding as a function of total IgG.
A competition assay using an anti-clonotypic antibody (mAb7.4) was used to detect the presence of anti-chromatin antibodies encoded by pTg18R. MAb7.4 is specific for paired heavy and light chain variable domains of the SN5-18R mAb. 96-well trays, coated with mAb7.4 and treated with blocking buffer, were incubated with test sera (1:100) together with biotin-SN5-18R (0.5 μg/ml) followed by streptavidin-Eu3+ and enhancement solution. 50% competition was attained with 0.15-0.3 mg/ml of unlabeled SN5-18R.
IgG anti-chromatin subclasses
IgH isotypes of anti-chromatin antibodies were determined as above, except that antibodies were detected using horse radish peroxidase-conjugated goat antibodies against IgG1, IgG2a, IgG2b and IgG3 (Southern Biotechnology Associates, Inc., Birmingham, AL) with the developing reagent, 2,2′-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid). Samples were defined positive when the absorbance at 405 nm exceeded the average of negative controls plus 3 standard deviations.
Results
Spontaneous disease in SWR mice that carry an IghV partial transgene
We generated three independent lines of mice (pTg18R) carrying an IghV partial transgene encoding the heavy chain variable domain of an antibody directed against a complex of H2A/H2B/dsDNA. The original hybridoma producing this antibody was produced from a spontaneously autoimmune (NZBxSWR)F1 female mouse and belonged to a large lineage (3). As show in Figure 1A, the partial transgene construct contains approximately 1 kb of DNA upstream of the leader sequence and approximately 1.6 kb of DNA downstream of the assembled JH segment but lacks all constant region sequences. As such, only rare B cells with a partial transgene that has translocated into the Igh locus can express an antibody heavy chain with a variable domain specified by the partial transgene. Previous studies have shown that translocation occurs by homologous recombination at the 3' end of the partial transgene (35). However, such recombination was so rare that it could not be detected in ex vivo B cells. Instead, recombination was revealed in B cell hybridomas that were selected to express the partial transgene by an immunization strategy. For each pTg18R line, the partial transgene was amplified from genomic DNA (Figure 1B) and sequenced to confirm that promoter and enhancer elements were intact (data not shown).
When the first cohort of pTg18R mice aged beyond 5 months, some of the mice developed visible signs of chronic inflammation, as manifested by skin lesions and a scruffy appearance (Figure 1C). Control SWR mice in our colony never showed these signs of disease. Anti-nuclear antibodies were also present in sera of some animals, as seen in stains of HEp-2 cells (Figure 1D) and in chromatin-binding immunoassays. All mice with visible evidence of disease or chromatin-binding antibodies also had enlarged lymph nodes and spleens (Figure 1E), but not to the extent that they could be seen from the exterior. Table 1 lists various disease manifestations distributed among affected individuals together with the age of onset and gender. These first data suggested that there was no female sex bias among affected mice, an interpretation confirmed by subsequent cohorts of pTg18R mice.
Table 1.
Manifestations of SLE-like disease in 3 lines of pTg18R mice
| Linea | Sex | Splenomegaly | Skin lesion |
IgG α-chrb | Agec |
|---|---|---|---|---|---|
| pTg18R.1 | M | + | − | − | 4.5 |
| pTg18R.1 | M | + | − | − | 9 |
| pTg18R.2 | M | − | + | + | 7 |
| pTg18R.2 | F | + | − | + | 7 |
| pTg18R.3 | M | + | − | − | 7.5 |
| pTg18R.3 | F | + | + | + | 5 |
Numbers after decimal point indicate independent lines of transgenic mice
IgG anti-chromatin detected in sera
Age in months at which first manifestation of disease was observed
SLE-like disease with kidney pathology
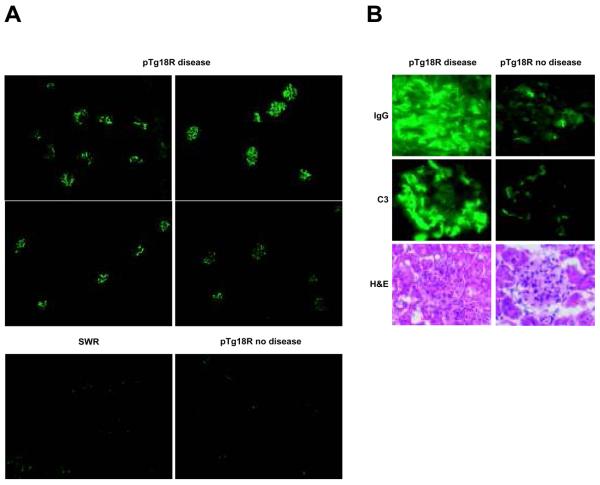
Because kidney pathology is a key manifestation of SLE, we examined kidneys of pTg18R mice that exhibited at least one other feature of disease (anti-chromatin IgG, skin lesions or splenomegaly). As predicted, most of these animals demonstrated some signs of kidney pathology, including glomerular deposits of IgG and/or C3 (Table 2 and Figure 2). However, proliferative disease was modest and limited to relatively few animals. This could be due to the fact that mice were sacrificed shortly after the first manifestation of disease. It is interesting to note that kidney pathology did not require the presence of IgG anti-chromatin, which has been seen in other studies (40, 41). To test for the presence of alternative autoantibodies that might induce pathology due to formation of immune complexes, we screen the sera of diseased animals for the presence of autoantibodies against small ribonucleoproteins (Sm), cardiolipin and erythrocytes. None of the diseased animals had detectable titers of autoantibodies against these self-antigens (data not shown). One animal without IgG or C3 deposition showed modest signs of kidney pathology. Although this animal did exhibit low levels of glomerular IgM deposition, they were not higher than that seen in several of the control nontransgenic SWR animals.
Table 2.
Kidney disease in pTg18R mice
| pTg18R diseaseda | IgG a-chrb | IgGc | IgMc | C3c | H&E staind |
|---|---|---|---|---|---|
| 1 | + | 1-2,M | 1,M | 0 | 0 |
| 2 | + | 0 | 1-2, M | 0 | 2, p, m |
| 3 | + | 3, M | 2-3, M,P | 3, M | 2, p, m, LI |
| 4 | + | 0 | 0 | 0 | 0 |
| 5 | − | 2, P | 3,P | 3, P | 2, p, m, LI |
| 6 | − | 0 | 1,M | 0 | 0 |
| 7 | + | 1-2, P | 2-3, M, P | 2, P | 3, p, m |
| 8 | − | 0 | 2,M | 2, M | 0 |
| 9 | + | 2, P, gbm | 0 | 1, P, gbm | 3, p, m |
| 10 | + | 1-2, M | 2, M | 1, M | 0 |
|
| |||||
| SWR | |||||
|
| |||||
| 1 | − | 0 | 1,M | 0 | 0 |
| 2 | − | 0 | 0 | 0 | 0 |
| 3 | − | 0 | 1,M | 0 | 0 |
| 4 | − | 0 | 2,M | 0 | 0 |
| 5 | − | 0 | 1,M | 0 | 0 |
| 6 | − | 0 | 0 | 0 | 0 |
| 7 | − | 0 | 0 | 0 | 0 |
| 8 | − | 0 | 0 | 0 | Nd |
| 9 | − | 0 | 0 | 0 | Nd |
| 10 | − | 0 | 0 | 0 | Nd |
These mice exhibited at least one of the 3 following signs of disease: IgG anti-chromatin, skin lesions or splenomegaly.
IgG anti-chromatin detected in sera.
Kidney sections were analyzed by immunofluorescence for IgG, IgM and C3 deposition. “M” indicates mesangial staining, “P” indicates peripheral capillary loop staining, “gbm” indicates staining clearly associated with glomerular basement membrane.
Kidney sections analyzed in an H&E stain. “p” indicates peripheral involvement, “m” indicates mesangial involvement and “LI” indicates inflammatory cellular infiltrates including macrophages, lymphocytes and neutrophils. “Nd” indicates not analyzed by H&E stain.
Numbers indicate pathologic index where 0 is no disease and 4 is most severe.
FIGURE 2.
Kidney pathology in autoimmune pTg18R mice. A, kidney sections stained with goat anti-mouse IgG-FITC at a low magnification (10x). B, IgG and complement factor 3 (C3) deposition revealed by immunofluorescence, and glomerular inflammation revealed by H&E staining for representative diseased (left panels) and non-diseased (right panels) pTg18R mice.
A CDR3 Arg is required for development of disease
Because disease developed in three independent lines of pTg18R mice and because it occurred in mice that were hemizygous with respect to the transgene, we infer that disease was not likely due to gene disruption at genomic sites of transgene integration. Given the origin of the partial transgene, we conjectured that development of disease was dependent upon the anti-nuclear specificity of antibody encoded by partial transgenes that recombined into the Igh locus. To test this idea we created three more lines of SWR mice with the same partial transgene in which a CDR3 Arg codon was converted to a serine codon. This alteration ablates the chromatin specificity of the original monoclonal antibody (37). As before, we amplified and sequenced the partial transgenes (pTg104RS) from genomic DNA to confirm that promoter and enhancer elements were intact.
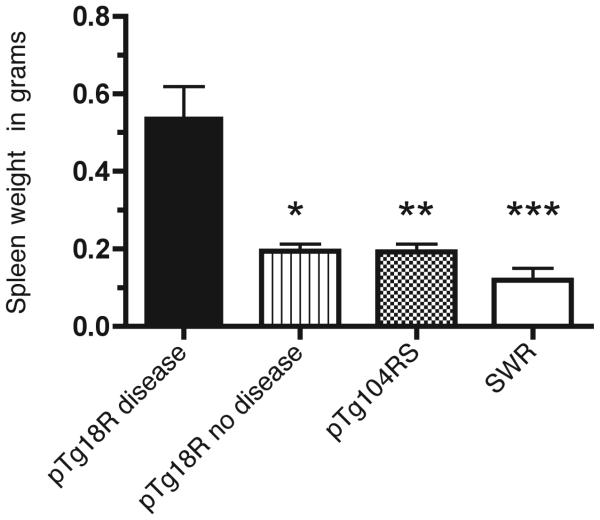
Several cohorts of pTg104RS mice and pTg18R and SWR mice were allowed to age until they developed disease, as assessed by the appearance or anti-chromatin antibodies, or until 12 months if no visible signs of disease were present. Animals were then sacrificed and their spleens were assessed for mass. While 23% of pTg18R mice showed clear signs of disease, none of the pTg104RS mice or SWR mice showed any sign at one year of age. There was no significant gender bias among afflicted pTg18R mice. Table 3 summarizes these results. The most common disease feature was splenomegaly followed by serum IgG anti-chromatin antibodies. On average, spleens of diseased pTg18R animals were 2.5 times the mass of those of pTg104RS or SWR mice or of non-diseased pTg18R mice (Figure 3). This was reflected by similar increases in lymphocyte numbers. No changes in the percentages of T and B cells subpopulations were seen in the spleens of diseased mice (data not shown). Although a modest shift favoring follicular B cells over the other B cell subsets was apparent, this was insufficient to account for the total increased splenic cellularity. We can infer from this that the >2-fold increase in cellularity must be due to increases in both T and B lymphocyte numbers. Kidneys of several aged pTg104RS were also analyzed for the presence of IgG deposits, but none showed any difference when compared to the SWR control mice (data not shown).
Table 3.
Frequency of SLE-like disease in pTg18R mice
| Disease | Spleenb | Lesion | IgG α-chrc | |
|---|---|---|---|---|
| pTg18R combined | 26/123 (21.13%) | 24 | 9 | 18 |
|
| ||||
| pTg18R.1a | 7/41 | 7 | 0 | 2 |
| pTg18R.2a | 5/18 | 3 | 3 | 4 |
| pTg18R.3a | 14/64 | 12 | 6 | 12 |
|
| ||||
| pTg104RS combined | 0/114 (0%) | 0 | 0 | 0 |
|
| ||||
| pTg104RS.1a | 0/56 | 0 | 0 | 0 |
| pTg104RS.2a | 0/41 | 0 | 0 | 0 |
| pTg104RS.3a | 0/17 | 0 | 0 | 0 |
|
| ||||
| SWR | 0/60 (0%) | 0 | 0 | 0 |
Numbers following decimal point indicate lines of pTg18R or pTg104RS mouse.
Splenomegaly
IgG anti-chromatin detected in sera.
FIGURE 3.
Splenomegaly in autoimmune pTg18R mice. Average spleen masses are indicated for diseased pTg18R mice, and non-diseased pTg18R, pTg104RS and SWR mice. The spleens of pTg18R diseased animals were significantly more massive than those the control groups (* pTg18R diseased vs pTg18R nondiseased p= 0.0167, ** pTg18R diseased vs pTg104RS p= 0.003, *** pTg18R diseased vs SWR p= 0.009).
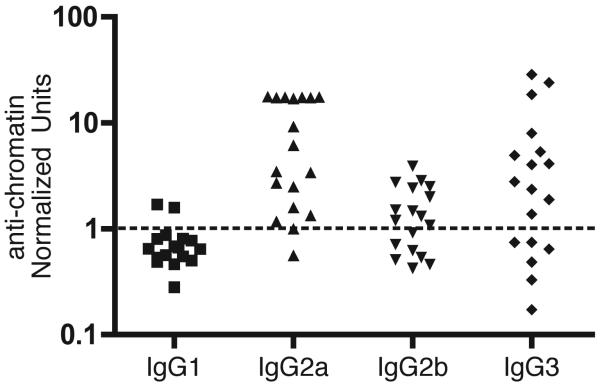
Chromatin-reactive autoantibodies
We tested the anti-chromatin IgG for heavy chain isotype and found that the predominant subclasses were γ2a and γ3, followed by γ2b (Figure 4). This subclass distribution, which is indicative of inflammation, is similar to that observed in other spontaneous models of SLE (1, 42). Sera of pTg18R mice that contained IgG anti-chromatin were further analyzed quantitatively in a fluoroimmunometric assay, as described in the Materials and Methods. The concentration of IgG anti-chromatin in these mice averaged 40 μg/ml, which was approximately 2-fold less than that in sera pooled from 10 autoimmune B6.Sle1 mice (Figure 5A). On average, pTg18R mice with IgG anti-chromatin also had total IgG concentrations that were approximately 2-fold higher than non-diseased and control animals (Figure 5B).
FIGURE 4.
Heavy chain isotypes of IgG anti-chromatin antibodies in pTg18R mice. IgG Anti-chromatin antibodies in pTg18R sera were quantified with isotype-specific antisera. 1 unit (dotted line) represents the average for a pool of negative sera from SWR mice plus 3 standard deviations.
To ensure that we were not misled by increased nonspecific binding to the assay trays due to increased concentrations of total IgG in diseased mice, we performed an additional test with serum IgG that was purified on a goat anti-mouse IgG affinity column. The purification procedure was designed to remove nuclear antigens from anti-nuclear antibodies because such immune complexes can produce artifacts in binding assays (37, 43, 44). Purified IgG was then tested at defined concentrations in the chromatin-binding assay. As shown in Figure 5C, the sera that tested positive for anti-chromatin IgG in the initial assay were positive again in the normalized assay using purified IgG. This result is consistent with idea that the anti-chromatin antibodies were products of specific clonal expansion, as opposed to polyclonal expansion/activation.
To determine if the anti-chromatin antibodies were encoded by the partial transgene, we tested sera of pTg18R mice in competition immunoassay using a monoclonal anti-idiotypic antibody directed against the original SN5-18 monoclonal antibody. However, no such antibodies were detected in any pTg18R mouse sera. In addition, we generated 4 hybridomas producing anti-chromatin antibodies from 2 diseased pTg18R mice and sequenced their heavy chain variable regions. The sequences revealed that none of the hybridomas expressed the VH gene encoded by pTg18R (data not shown). Thus, we found that most diseased pTg18R mice produced anti-chromatin antibodies, but none of the mice had detectable levels of antibody derived from the partial transgene.
Discussion
We describe an autoimmune disease that develops spontaneously in SWR mice that carry a partial transgene encoding the heavy chain V domain of an anti-nuclear antibody with specificity for a complex of H2A/H2B/dsDNA. The disease is variously manifested among individuals by one or more of the following features: serum anti-chromatin antibodies, kidney pathology, skin lesions and enlarged secondary lymphoid organs, which collectively occur at a penetrance of ~20-25%. A majority of the anti-nuclear antibodies were of heavy chain isotypes (γ2a, γ3) indicative of inflammation and class switch recombination driven by IFN-γ. This, together with the other disease manifestations, such as skin lesions and enlarged lymphoid organs, indicate an inflammatory condition.
The kidney pathology observed in our mice is milder than that seen in some of the other prototypical SLE disease models and does not strongly correlate with the presence of IgG anti-chromatin antibodies. One possible explanation is that our animals were sacrificed soon after any sign of disease was observed, and before sufficient time elapsed for severe kidney pathology to develop. In addition, the presence of anti-nuclear antibodies alone isn't always accompanied by kidney disease, as other factors such as genetic predisposition and inflammatory milieu are also important (45). The SWR strain appears to be relatively resistant to antibodies directed to the glomerular basement membrane (46).
None of the autoimmune features observed in pTg18R mice were observed in similarly aged control wildtype SWR mice, which are not genetically-predisposed to develop autoimmunity. Moreover, it is unlikely that disease in pTg18R mice is due a genetic disruption at the site of transgene integration because disease occurred in hemizygous pTg18R mice, in all 3 lines of pTg18R mice, and not in any of 3 control pTg104RS lines. These observations indicate that pTg18R induces autoimmunity in mice that are not genetically predisposed.
We infer that protein product of the partial transgene initiates disease because a VHCDR3 arginine codon in pTg18R was required for pathology. This same Arg was also required for the chromatin-specificity of the original anti-nuclear monoclonal antibody that defines our system (37). Moreover, the SWR genome carries the same Vκ10.2 gene that encodes the light chain V region of the original anti-chromatin monoclonal antibody (47).
It is unlikely that the untransloctated pTg18R directs synthesis of a protein product because mRNA lacking proper termination and polyadenylation sequences is highly unstable. We also considered the possibility that pTg18R integrated within the genome in such a manner as to encode a pathogenic fusion protein, but dismissed this for two reasons. First, such an event would have to occur 3 times independently. Second, the recombination event(s) would have to be restricted to a 70 base segment of DNA between the disease-determining Arg codon at position 104 in CDR3 and an in-frame translation termination codon located just 12 bases downstream of the last JH codon. We considered the possibility that the NZB-derived pTg18R encoded an immunogenic VHCDR3 peptide (containing Arg104) with respect to SWR CD4+ T cells and hence provided an avenue of help to B cells without recombining into the Igh locus. This is also highly unlikely because of the mRNA instability issue mentioned above. Moreover, in a preceding study, immunodominant VH peptides of the 18R antibody (restricted by the SWR I-Aq molecule) were found in FR1 and CDR2 (47). Because these peptides are also encoded by pTg104RS, we would have expected autoimmunity in pTg104RS mice if T cell help to VH peptides were rate-limiting in disease initiation.
IghV partial Tg mice are unique among current Ig transgene models for autoimmunity because genetic recombination is presumably required for expression of the heavy chain variable domain encoded by the transgene. Importantly, partial transgenes have no heptamer/nonamer recombination signal sequences, and studies in the anti-arsonate model have confirmed that partial transgenes recombine into the Igh locus by homologous recombination (33-36). In principle, such recombination could occur at any stage of B cell development, even in a cell that already expresses an antigen receptor. The result of such a scenario would be similar to one in which a mature nontransgenic B cell generates an autoreactive antibody by the process of somatic hypermutation or receptor editing in the periphery (48-52). Evidence for receptor editing in peripheral B cells of autoimmune-prone nontransgenic mice was recently reported by Wakui et al. (53) and in autoimmune-prone Ig transgenic mice by two laboratories (54, 55).
Late-stage recombination of pTg18R into the Igh locus of peripheral B cells could explain why pTg18R mice develop autoimmunity despite the infrequency of such recombination events. Presumably these B cells would not have to traverse all stages of development and face the associated self-tolerance checkpoints that begin in the bone marrow. Late-stage recombination could also explain why there is no gender bias for disease in pTg18R mice. Estrogen relaxes negative selection of autoreactive B cells and is partly responsible for the female sex bias seen in spontaneous human SLE or animal models of SLE (56, 57). However, estrogen may not be so critical if the B cell is already activated at the time when partial transgene rearrangement occurs. Regardless of why partial transgene mice develop autoimmunity, the induction of disease by a partial Ig transgene in mice that are not otherwise predisposed to autoimmunity is unique among current models of SLE.
Our findings suggest that immunocompetent precursors to anti-nuclear B cells can be rate-limiting with respect to the development of anti-nuclear antibodies in systemic autoimmunity. Although this idea is not widely accepted by investigators of SLE, there is some support for it in the literature. For example, Wang et al. (58) reported that a DNA mimetope did not induce anti-nuclear antibodies in DBA/2 mice, although it did so in BALB/c mice. Similarly, we have found that when SWR mice are injected with calf chromatin, they develop strong IgG anti-chromatin antibodies that bind calf chromatin but not mouse chromatin (unpublished data). Both of these observations indicate that even though T cell help was available, anti-nuclear B cells could not be successfully recruited into an autoimmune response in mice of certain genetic dispositions. There are many possible reasons for this, including the absence of anti-nuclear B cells in mice of certain genetic compositions.
Partial transgenes translocate to the Igh locus at a very low frequency, such that very few B cells in pTg18R mice are expected to produce a receptor with the appropriate light chain that confers specificity for H2A/H2B/dsDNA. In fact, like Guisti et al. (35), we have not been able to detect the recombined partial transgene in central or peripheral lymphoid tissue by PCR of ex-vivo lymphocytes. Moreover, in another study involving a partial transgene encoding the VH of a rheumatoid factor crossed onto an MRL/lpr genetic background, the pTg-derived antibody could not be detected even though the repertoire of spontaneous autoantibodies was clearly altered (59). The low frequency of recombination could, in part, explain the low penetrance and delayed kinetics of disease in pTg18R mice. In addition, other regulatory mechanisms may functionally inactivate or physically remove most of the anti-nuclear B cells expressing the partial transgene. Hahn and colleagues reported evidence for regulatory CD8 T cells that are able to suppress autoreactive B cells, apparently in a manner that is specific for the B cell receptor (60). Cells of this type in the SWR strain conceivably could inhibit anti-nuclear B cells expressing the pTg18R, resulting in marginal propagation of the B cells and a low penetrance of disease. Although some of our diseased pTg18R mice produced no detectable serum anti-nuclear antibodies, there are controversial reports of seronegative SLE, in which no anti-nuclear antibodies can be detected. This was seen humans and in an MRL lpr/lpr mouse model where B cells express a transgene-encoded Ig that cannot be secreted (40, 61, 62). It is also possible that the combination of the pTg18R heavy chain with an endogenous light chain generated a tissue-specific autoreactive antibody with a nonnuclear specificity that induced the pathology seen in some of our mice without anti-chromatin antibodies.
Collectively, our results support the idea that low frequencies of anti-nuclear B cells can initiate autoimmunity with SLE-like manifestations in animals that are not genetically predisposed. They show that the product of the Ig transgene that initiates disease is not a major contributor to the anti-nuclear antibodies observed at late stages of disease. And they show that in some cases, the transgene product induces what appears to be a seronegative form of autoimmunity. Whether anti-nuclear antibodies or B cells cryptically initiate spontaneous SLE is unknown, but the possibility is raised by the apparent seronegative feature of disease that is shared between some of our pTg18R mice and certain patients that ostensibly have SLE.
Supplementary Material
Acknowledgments
We thank Dr. Edward Wakeland for providing B6.Sle1 mice and Ryan Heiser and Dr. Katja Aviszus for their helpful suggestions. This work was supported by Grants from the National Institutes of Health, AI033613 and AI048108.
Abbreviations
- SLE
systemic lupus erythematosus
- pTg
partial transgene
References
- 1.Kotzin BL. Systemic lupus erythematosus. Cell. 1996;85(3):303–6. doi: 10.1016/s0092-8674(00)81108-3. [DOI] [PubMed] [Google Scholar]
- 2.Shlomchik M, Mascelli M, Shan H, Radic MZ, Pisetsky D, Marshak-Rothstein A, et al. Anti-DNA antibodies from autoimmune mice arise by clonal expansion and somatic mutation. J Exp Med. 1990;171(1):265–92. doi: 10.1084/jem.171.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Portanova JP, Creadon G, Zhang X, Smith DS, Kotzin BL, Wysocki LJ. An early post-mutational selection event directs expansion of autoreactive B cells in murine lupus. Mol Immunol. 1995;32(2):117–35. doi: 10.1016/0161-5890(94)00129-o. [DOI] [PubMed] [Google Scholar]
- 4.Diamond B, Katz JB, Paul E, Aranow C, Lustgarten D, Scharff MD. The role of somatic mutation in the pathogenic anti-DNA response. Annu Rev Immunol. 1992;10:731–57. doi: 10.1146/annurev.iy.10.040192.003503. [DOI] [PubMed] [Google Scholar]
- 5.Wellmann U, Letz M, Herrmann M, Angermuller S, Kalden JR, Winkler TH. The evolution of human anti-double-stranded DNA autoantibodies. Proc Natl Acad Sci U S A. 2005;102(26):9258–63. doi: 10.1073/pnas.0500132102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohan C, Adams S, Stanik V, Datta SK. Nucleosome: a major immunogen for pathogenic autoantibody-inducing T cells of lupus. J Exp Med. 1993;177(5):1367–81. doi: 10.1084/jem.177.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaliyaperumal A, Mohan C, Wu W, Datta SK. Nucleosomal peptide epitopes for nephritis-inducing T helper cells of murine lupus. J Exp Med. 1996;183(6):2459–69. doi: 10.1084/jem.183.6.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X, Smith DS, Guth A, Wysocki LJ. A receptor presentation hypothesis for T cell help that recruits autoreactive B cells. J Immunol. 2001;166(3):1562–71. doi: 10.4049/jimmunol.166.3.1562. [DOI] [PubMed] [Google Scholar]
- 9.Singh RR, Kumar V, Ebling FM, Southwood S, Sette A, Sercarz EE, et al. T cell determinants from autoantibodies to DNA can upregulate autoimmunity in murine systemic lupus erythematosus. J Exp Med. 1995;181(6):2017–27. doi: 10.1084/jem.181.6.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snyder CM, Aviszus K, Heiser RA, Tonkin DR, Guth A, Wysocki LJ. Activation and Tolerance in CD4+ T Cells Reactive to an Immunoglobulin Variable Region. Journal of Experimental Medicine. 2004;200(1):1–11. doi: 10.1084/jem.20031234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gleichman H. Systemic lupus erythematous triggered by diphenylhydantoin. Arthritis Rheum. 1982;25(11):1387–8. doi: 10.1002/art.1780251121. [DOI] [PubMed] [Google Scholar]
- 12.Desai DD, Krishnan MR, Swindle JT, Marion TN. Antigen-specific induction of antibodies against native mammalian DNA in nonautoimmune mice. J Immunol. 1993;151(3):1614–26. [PubMed] [Google Scholar]
- 13.Putterman C, Deocharan B, Diamond B. Molecular analysis of the autoantibody response in peptide-induced autoimmunity. J Immunol. 2000;164(5):2542–9. doi: 10.4049/jimmunol.164.5.2542. [DOI] [PubMed] [Google Scholar]
- 14.Hahn BH, Singh RR, Ebling FM. Self Ig peptides that help anti-DNA antibody production: importance of charged residues. Lupus. 1998;7(5):307–13. doi: 10.1191/096120398678920145. [DOI] [PubMed] [Google Scholar]
- 15.Seo SJ, Fields ML, Buckler JL, Reed AJ, Mandik-Nayak L, Nish SA, et al. The impact of T helper and T regulatory cells on the regulation of anti-double-stranded DNA B cells. Immunity. 2002;16(4):535–46. doi: 10.1016/s1074-7613(02)00298-4. [DOI] [PubMed] [Google Scholar]
- 16.Sekiguchi DR, Jainandunsing SM, Fields ML, Maldonado MA, Madaio MP, Erikson J, et al. Chronic graft-versus-host in Ig knockin transgenic mice abrogates B cell tolerance in anti-double-stranded DNA B cells. J Immunol. 2002;168(8):4142–53. doi: 10.4049/jimmunol.168.8.4142. [DOI] [PubMed] [Google Scholar]
- 17.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301(5638):1374–7. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 18.Striebich CC, Miceli RM, Schulze DH, Kelsoe G, Cerny J. Antigen-binding repertoire and Ig H chain gene usage among B cell hybridomas from normal and autoimmune mice. J Immunol. 1990;144(5):1857–65. [PubMed] [Google Scholar]
- 19.Coutinho A, Kazatchkine MD, Avrameas S. Natural autoantibodies. Curr Opin Immunol. 1995;7(6):812–8. doi: 10.1016/0952-7915(95)80053-0. [DOI] [PubMed] [Google Scholar]
- 20.Lydyard PM, Quartey-Papafio R, Broker B, Mackenzie L, Jouquan J, Blaschek MA, et al. The antibody repertoire of early human B cells. I. High frequency of autoreactivity and polyreactivity. Scand J Immunol. 1990;31(1):33–43. doi: 10.1111/j.1365-3083.1990.tb02740.x. [DOI] [PubMed] [Google Scholar]
- 21.Marion TN, Krishnan MR, Steeves MA, Desai DD. Affinity maturation and autoimmunity to DNA. Curr Dir Autoimmun. 2003;6:123–53. doi: 10.1159/000066859. [DOI] [PubMed] [Google Scholar]
- 22.Verkoczy LK, Martensson AS, Nemazee D. The scope of receptor editing and its association with autoimmunity. Curr Opin Immunol. 2004;16(6):808–14. doi: 10.1016/j.coi.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 23.Goodnow CC, Sprent J, Fazekas de St Groth B, Vinuesa CG. Cellular and genetic mechanisms of self tolerance and autoimmunity. Nature. 2005;435(7042):590–7. doi: 10.1038/nature03724. [DOI] [PubMed] [Google Scholar]
- 24.Yachimovich N, Mostoslavsky G, Yarkoni Y, Verbovetski I, Eilat D. The efficiency of B cell receptor (BCR) editing is dependent on BCR light chain rearrangement status. European Journal of Immunology. 2002;32(4):1164–74. doi: 10.1002/1521-4141(200204)32:4<1164::AID-IMMU1164>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 25.Mandik-Nayak L, Seo SJ, Sokol C, Potts KM, Bui A, Erikson J. MRL-lpr/lpr mice exhibit a defect in maintaining developmental arrest and follicular exclusion of anti-double-stranded DNA B cells. J Exp Med. 1999;189(11):1799–814. doi: 10.1084/jem.189.11.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Li H, Ni D, Weigert M. Anti-DNA B cells in MRL/lpr mice show altered differentiation and editing pattern. Journal of Experimental Medicine. 2002;196(12):1543–52. doi: 10.1084/jem.20021560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steeves MA, Marion TN. Tolerance to DNA in (NZB x NZW)F1 mice that inherit an anti-DNA V(H) as a conventional micro H chain transgene but not as a V(H) knock-in transgene. J Immunol. 2004;172(11):6568–77. doi: 10.4049/jimmunol.172.11.6568. [DOI] [PubMed] [Google Scholar]
- 28.Sekiguchi DR, Yunk L, Gary D, Charan D, Srivastava B, Allman D, et al. Development and selection of edited B cells in B6.56R mice. J Immunol. 2006;176(11):6879–87. doi: 10.4049/jimmunol.176.11.6879. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Li L, Kumar KR, Xie C, Lightfoot S, Zhou XJ, et al. Lupus susceptibility genes may breach tolerance to DNA by impairing receptor editing of nuclear antigen-reactive B cells. J Immunol. 2007;179(2):1340–52. doi: 10.4049/jimmunol.179.2.1340. [DOI] [PubMed] [Google Scholar]
- 30.Prak EL, Trounstine M, Huszar D, Weigert M. Light chain editing in kappa-deficient animals: a potential mechanism of B cell tolerance. J Exp Med. 1994;180(5):1805–15. doi: 10.1084/jem.180.5.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roark JH, Kuntz CL, Nguyen KA, Mandik L, Cattermole M, Erikson J. B cell selection and allelic exclusion of an anti-DNA Ig transgene in MRL-lpr/lpr mice. J Immunol. 1995;154(9):4444–55. [PubMed] [Google Scholar]
- 32.Iliev A, Spatz L, Ray S, Diamond B. Lack of allelic exclusion permits autoreactive B cells to escape deletion. J Immunol. 1994;153(8):3551–6. [PubMed] [Google Scholar]
- 33.Giusti AM, Coffee R, Manser T. Somatic recombination of heavy chain variable region transgenes with the endogenous immunoglobulin heavy chain locus in mice. Proc Natl Acad Sci U S A. 1992;89(21):10321–5. doi: 10.1073/pnas.89.21.10321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vora KA, Manser T. Altering the antibody repertoire via transgene homologous recombination: evidence for global and clone-autonomous regulation of antigen-driven B cell differentiation. J Exp Med. 1995;181(1):271–81. doi: 10.1084/jem.181.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giusti AM, Manser T. Somatic generation of hybrid antibody H chain genes in transgenic mice via interchromosomal gene conversion. J Exp Med. 1994;179(1):235–48. doi: 10.1084/jem.179.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giusti AM, Manser T. Hypermutation is observed only in antibody H chain V region transgenes that have recombined with endogenous immunoglobulin H DNA: implications for the location of cis-acting elements required for somatic mutation. J Exp Med. 1993;177(3):797–809. doi: 10.1084/jem.177.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guth AM, Zhang X, Smith D, Detanico T, Wysocki LJ. Chromatin specificity of anti-double-stranded DNA antibodies and a role for arg residues in the third complementarity-determining region of the heavy chain. J Immunol. 2003;171(11):6260–6. doi: 10.4049/jimmunol.171.11.6260. [DOI] [PubMed] [Google Scholar]
- 38.Gefter ML, Margulies DH, Scharff MD. A simple method for polyethylene glycol-promoted hybridization of mouse myeloma cells. Somatic Cell Genet. 1977;3(2):231–6. doi: 10.1007/BF01551818. [DOI] [PubMed] [Google Scholar]
- 39.Keelan JA, France JT, Barling PM. An alternative fluorescence enhancement solution for use in lanthanide-based time-resolved fluoroimmunoassays. Clin Chem. 1987;33(12):2292–5. [PubMed] [Google Scholar]
- 40.Chan OT, Hannum LG, Haberman AM, Madaio MP, Shlomchik MJ. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J Exp Med. 1999;189(10):1639–48. doi: 10.1084/jem.189.10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Christensen SR, Kashgarian M, Alexopoulou L, Flavell RA, Akira S, Shlomchik MJ. Toll-like receptor 9 controls anti-DNA autoantibody production in murine lupus. J Exp Med. 2005;202(2):321–31. doi: 10.1084/jem.20050338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fisher CL, Eisenberg RA, Cohen PL. Quantitation and IgG subclass distribution of antichromatin autoantibodies in SLE mice. Clinical Immunology & Immunopathology. 1988;46(2):205–13. doi: 10.1016/0090-1229(88)90183-3. [DOI] [PubMed] [Google Scholar]
- 43.Kramers C, Hylkema MN, van Bruggen MC, van de Lagemaat R, Dijkman HB, Assmann KJ, et al. Anti-nucleosome antibodies complexed to nucleosomal antigens show anti-DNA reactivity and bind to rat glomerular basement membrane in vivo. J Clin Invest. 1994;94(2):568–77. doi: 10.1172/JCI117371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mason LJ, Lambrianides A, Haley JD, Manson JJ, Latchman DS, Isenberg DA, et al. Stable expression of a recombinant human antinucleosome antibody to investigate relationships between antibody sequence, binding properties, and pathogenicity. Arthritis Res Ther. 2005;7(5):R971–83. doi: 10.1186/ar1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu T, Mohan C. Three pathogenic determinants in immune nephritis--anti-glomerular antibody specificity, innate triggers and host genetics. Front Biosci. 2007;12:2207–11. doi: 10.2741/2223. [DOI] [PubMed] [Google Scholar]
- 46.Xie C, Sharma R, Wang H, Zhou XJ, Mohan C. Strain distribution pattern of susceptibility to immune-mediated nephritis. J Immunol. 2004;172(8):5047–55. doi: 10.4049/jimmunol.172.8.5047. [DOI] [PubMed] [Google Scholar]
- 47.Guo W, Smith D, Guth A, Aviszus K, Wysocki LJ. T cell tolerance to germline-encoded antibody sequences in a lupus-prone mouse. J Immunol. 2005;175(4):2184–90. doi: 10.4049/jimmunol.175.4.2184. [DOI] [PubMed] [Google Scholar]
- 48.Hertz M, Kouskoff V, Nakamura T, Nemazee D. V(D)J recombinase induction in splenic B lymphocytes is inhibited by antigen-receptor signalling. Nature. 1998;394(6690):292–5. doi: 10.1038/28419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han S, Dillon SR, Zheng B, Shimoda M, Schlissel MS, Kelsoe G. V(D)J recombinase activity in a subset of germinal center B lymphocytes. Science. 1997;278(5336):301–5. doi: 10.1126/science.278.5336.301. see comments. [DOI] [PubMed] [Google Scholar]
- 50.Hikida M, Nakayama Y, Yamashita Y, Kumazawa Y, Nishikawa SI, Ohmori H. Expression of recombination activating genes in germinal center B cells: involvement of interleukin 7 (IL-7) and the IL-7 receptor. J Exp Med. 1998;188(2):365–72. doi: 10.1084/jem.188.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hikida M, Ohmori H. Rearrangement of lambda light chain genes in mature B cells in vitro and in vivo. Function of reexpressed recombination-activating gene (RAG) products. J Exp Med. 1998;187(5):795–9. doi: 10.1084/jem.187.5.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Monroe RJ, Seidl KJ, Gaertner F, Han S, Chen F, Sekiguchi J, et al. RAG2:GFP knockin mice reveal novel aspects of RAG2 expression in primary and peripheral lymphoid tissues. Immunity. 1999;11(2):201–12. doi: 10.1016/s1074-7613(00)80095-3. [DOI] [PubMed] [Google Scholar]
- 53.Wakui M, Kim J, Butfiloski EJ, Morel L, Sobel ES. Genetic dissection of lupus pathogenesis: Sle3/5 impacts IgH CDR3 sequences, somatic mutations, and receptor editing. J Immunol. 2004;173(12):7368–76. doi: 10.4049/jimmunol.173.12.7368. [DOI] [PubMed] [Google Scholar]
- 54.Brard F, Shannon M, Prak EL, Litwin S, Weigert M. Somatic mutation and light chain rearrangement generate autoimmunity in anti-single-stranded DNA transgenic MRL/lpr Mice. J Exp Med. 1999;190(5):691–704. doi: 10.1084/jem.190.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yarkoni Y, Fischel R, Kat I, Yachimovich-Cohen N, Eilat D. Peripheral B cell receptor editing may promote the production of high-affinity autoantibodies in CD22-deficient mice. Eur J Immunol. 2006;36(10):2755–67. doi: 10.1002/eji.200636190. [DOI] [PubMed] [Google Scholar]
- 56.Cohen-Solal JF, Jeganathan V, Grimaldi CM, Peeva E, Diamond B. Sex hormones and SLE: influencing the fate of autoreactive B cells. Curr Top Microbiol Immunol. 2006;305:67–88. doi: 10.1007/3-540-29714-6_4. [DOI] [PubMed] [Google Scholar]
- 57.Grimaldi CM, Jeganathan V, Diamond B. Hormonal regulation of B cell development: 17 beta-estradiol impairs negative selection of high-affinity DNA-reactive B cells at more than one developmental checkpoint. J Immunol. 2006;176(5):2703–10. doi: 10.4049/jimmunol.176.5.2703. [DOI] [PubMed] [Google Scholar]
- 58.Wang C, Khalil M, Ravetch J, Diamond B. The naive B cell repertoire predisposes to antigen-induced systemic lupus erythematosus. J Immunol. 2003;170(9):4826–32. doi: 10.4049/jimmunol.170.9.4826. [DOI] [PubMed] [Google Scholar]
- 59.Hande S, Jacobson BA, Manser T. A recurrent clonotype in the spontaneous anti-IgG2a rheumatoid factor response of lpr/lpr mice. J Immunol. 1996;156(5):1856–64. [PubMed] [Google Scholar]
- 60.Singh RR, Ebling FM, Albuquerque DA, Saxena V, Kumar V, Giannini EH, et al. Induction of autoantibody production is limited in nonautoimmune mice. J Immunol. 2002;169(1):587–94. doi: 10.4049/jimmunol.169.1.587. [DOI] [PubMed] [Google Scholar]
- 61.Meulders Q, Michel C, Marteau P, Grange JD, Mougenot B, Ronco P, et al. Association of chronic interstitial cystitis, protein-losing enteropathy and paralytic ileus with seronegative systemic lupus erythematosus: case report and review of the literature. Clin Nephrol. 1992;37(5):239–44. [PubMed] [Google Scholar]
- 62.Ozdemir FN, Elsurer R, Akcay A, Ozdemir BH, Sezer S, Kuscu E, et al. Seronegative systemic lupus erythematosus: etiology of nephrotic syndrome and acute renal failure in early postpartum period. Lupus. 2005;14(8):629–31. doi: 10.1191/0961203305lu2148cr. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.