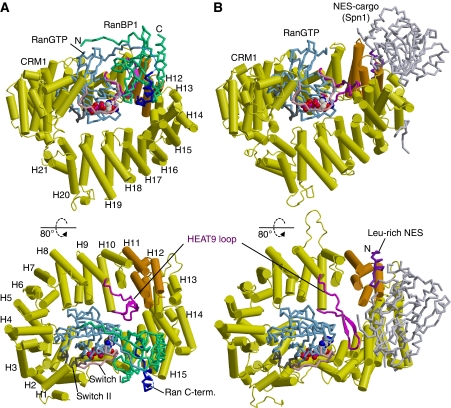
Figure 2.
Overview of crystal structures. (A) Yeast CRM1:RanBP1:RanGTP complex (this study; PDB accession code: 3M1I). (B) CRM1:Spn1:RanGTP complex (Monecke et al, 2009; PDB accession code: 3GJX). The HEAT repeats of CRM1 are labelled H1–H21. CRM1 is coloured in yellow, except that HEAT9 loop (that shows large movement between the two structures) and HEAT repeats 11–12 (that constitute the NES-binding site) are highlighted in magenta and orange, respectively. Ran is coloured in cyan (switch I, switch II and the C-terminal tail are highlighted in pink, grey and blue, respectively; GTP is shown as space-filling spheres). RanBP1 and Spn1 are coloured in green and light grey, respectively. Leu-rich NES at the N-terminus of Spn1 (residues 1–16), highlighted in purple, binds to the outer surface of CRM1, away from Ran, whereas RanBP1 makes intimate contacts with RanGTP and binds to a site that is distinct from the NES-binding site. The structures suggest that RanBP1, together with the C-terminal tail of Ran, induces a large movement of HEAT9 loop that in turn causes conformational rearrangements in HEAT repeats 11–12 so that the hydrophobic NES-binding cleft on the CRM1 outer surface closes, squeezing out the NES-cargo (see Figures 3 and 4 and Supplementary Movies S1–S3 for details).