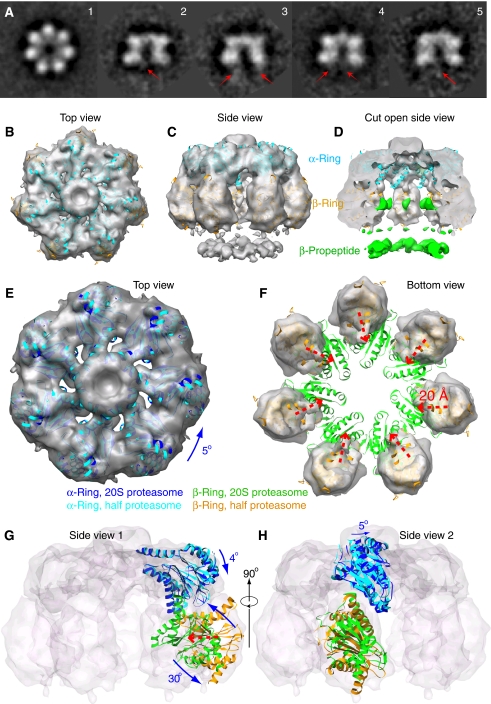
Figure 1.
The 3D cryo-EM structure of the Mtb half proteasome. (A) A gallery of 2D class averages of the cryo-EM images of the Mtb half proteasome particles. The red arrows in the side views (panels 2–5) indicate averaged residual density of the flexible β-propeptides located at the bottom under the β-ring. (B–D) Surface rendered cryo-EM 3D map of the Mtb half proteasome docked individually with the α-subunit (in cyan) and β-subunit (in brown) crystal structures in the top, side, and cut-open views. The EM density map is shown partially transparent. The discontinuous densities, coloured green in the cut-open view, are attributed to the flexible β-propeptides, and disappear at display threshold higher than 1.4 σ (D). (E, F) Computationally segmented α-ring (E) and β-ring electron density (F), superimposed with individually docked α-subunits (E, cyan) and β-subunits (F, yellow). Also superimposed are the α-ring (in blue) and β-ring structure (in green) of the mature Mtb 20S proteasome to illustrate the required structural re-arrangement. (G, H) Two orthogonal side views with electron density map shown as transparent surface rendering in grey. One α-subunit and one β-subunit structure in half proteasome and full proteasome positions are shown in carton representation. (E–H) The translation and rotation of the individual α- and β-subunits in the Mtb half proteasome required for reaching the full Mtb 20S proteasome structure. See text for details.