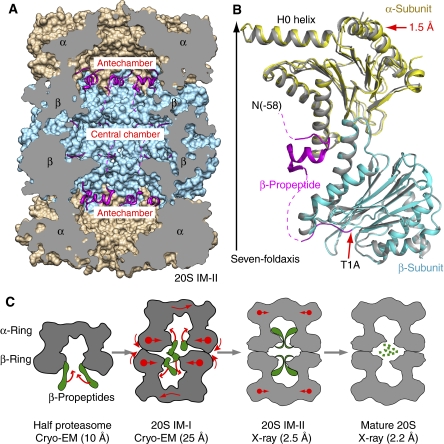
Figure 3.
The β-propeptide is ordered and ascended to the antechambers in the 20S T1A mutant proteasome (20S IM-II). (A) The crystal structure of the Mtb 20S IM-II shown in a cut-open side view. The β-propepetides are shown as ribbons in magenta, and the α- and β-rings are rendered in surface view and shown in brown and cyan, respectively. (B) Superposition of one α/β heterodimer of 20S IM-II (in yellow and cyan, respectively) with that of the mature Mtb 20S proteasome (in grey). The β-propeptide is shown in magenta. The dashed curves indicate unresolved residues. (C) Proposed structural changes accompanying the assembly of the Mtb 20S proteasome, starting from the half proteasome to the metastable 20S assembly intermediate I (20S IM-I), towards the assembled but immature proteasome intermediate II as represented by the T1A mutant structure (20S IM-II), and finally to the mature 20S proteasome. The β-propeptides are illustrated in green.