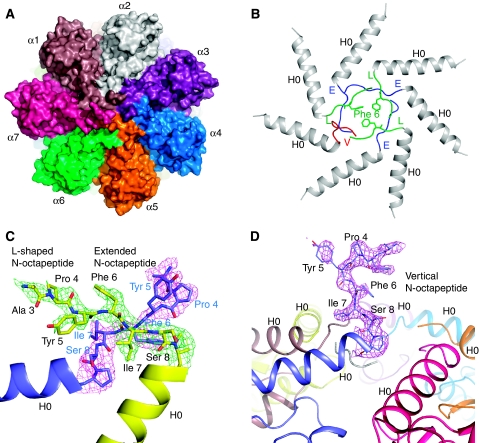
Figure 4.
The Mtb proteasome has a tightly closed gate in the crystal structure of the T1A mutant 20S proteasome. (A) A space-filling surface view of the T1A mutant 20S proteasome shows that the substrate entrance channel in the centre of the top view is totally sealed. (B) Ribbon representation of the end structure of the T1A mutant 20S. The α-octapeptides take the ‘E' (coloured in blue) and ‘L' conformation (coloured in green) alternatively, except for the last one that protrudes upwards (labelled as ‘V' conformation, coloured in red), being almost perpendicular to the end surface of the Mtb 20S cylinder. (C) The 2Fo−Fc electron density of the α-octapeptides in two adjacent α-subunits rendered at 1.0 σ. The ‘L' configuration is in green, and the ‘E' configuration in purple. This picture is viewed from the top of the proteasome cylinder. Note residue Phe-3 is modelled as Ala-3 because of the absence of the side chain density. (D) The α-octapeptide that assumes the up-standing (‘V') conformation. Superimposed in purple is its 2Fo−Fc density contoured at 1.0 σ. The residue Ser-2 is modelled as Ala-2 because of the absence of the side chain density. The picture is viewed from the side of the proteasome cylinder.