Figure 5.
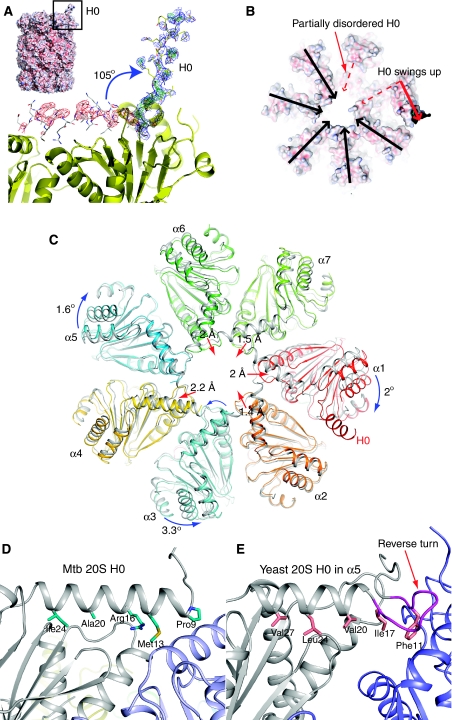
The H0 helix in one α-subunit is displaced in the crystal structure of the Mtb 20SOG. (A) The electron density map of the displaced H0 helix, which is shown in the yellow stick-and-ball mode. The normal H0 position is shown in the grey stick-and-ball mode. The electron density map was calculated using a model with H0 in its normal position. The green and red meshes are Fo−Fc different density map contoured at ±3.0 σ, respectively, and the blue mesh is 2Fo−Fc electron density map contoured at 1.0 σ. Inserted at the top left corner is a surface representation of the Mtb 20SOG. (B) Top surface view of the Mtb 20SOG. The upward swinging of H0 helix in one α-subunit (dashed and solid red arrows) results in partial disorder of the H0 in its neighbouring α-subunit (dashed red arrow). (C) Multiple subunit movements in the α-ring of the 20SOG structure (in colour) as shown by comparing with the WT 20S α-ring (in grey). The α-subunit harbouring the displaced H0 helix (labelled α1) shifted outwards by ∼2 Å (the red arrow head), and rotated clockwise 2° (the blue curved arrow). In response to the outward movement of the α1-subunit the neighbouring α2-, α7-, and α6-subunits shifted towards the centre (the red arrows). Finally, responding to the changes at the right side of the α-ring (α1, α2, α6, and α7), the α5 and α3 at the left side of the ring each slightly rotated in an opposite direction, and the α4-subunit moved outwards by 2 Å. (D) The interactions of the H0 helix with the underlying structure at its normal position in the Mtb 20S proteasome as compared with the H0 helix in the α5-subunit of the yeast 20S proteasome (E). The reverse turn in the yeast α-subunit, which is absent in the Mtb α-subunit, is shown in purple in (E).