Abstract
Upper gastrointestinal (GI) cancers of the stomach and esophagus have high incidence and mortality worldwide, but they are uncommon in Western countries. Little information exists on the association between vitamin D and risk of upper GI cancers. This study examined the association between circulating 25-hydroxyvitamin D (25(OH)D) and upper GI cancer risk in the Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Concentrations of 25(OH)D were measured from 1,065 upper GI cancer cases and 1,066 age-, sex-, race-, and season-of blood draw–matched controls from 8 prospective cohort studies. In multivariate-adjusted models, circulating 25(OH)D concentration was not significantly associated with upper GI cancer risk. Subgroup analysis by race showed that among Asians, but not Caucasians, lower concentrations of 25(OH)D (<25 nmol/L) were associated with a statistically significant decreased risk of upper GI cancer (reference: 50–<75 nmol/L) (odds ratio = 0.53, 95% confidence interval: 0.31, 0.91; P trend = 0.003). Never smokers with concentrations of <25 nmol/L showed a lower risk of upper GI cancers (odds ratio = 0.55, 95% confidence interval: 0.31, 0.96). Subgroup analyses by alcohol consumption produced opposing trends. Results do not support the hypothesis that interventions aimed at increasing vitamin D status would lead to a lower risk of these highly fatal cancers.
Keywords: case-control studies, cohort studies, esophageal neoplasms, prospective studies, stomach neoplasms, vitamin D
Upper gastrointestinal (GI) cancers of the stomach and esophagus have high incidence and mortality worldwide, but they are uncommon in Western countries. Few epidemiologic studies have examined the association between vitamin D and risk of upper GI cancers of the esophagus or stomach. The major source of vitamin D for most people is generation through the skin during exposure to ultraviolet B radiation, whereas diet contributes little, especially among those who do not consume vitamin D–fortified products or oily fish. Vitamin D can be antiproliferative in cells of the skin, colon, breast, and prostate, among others, and may also limit proinflammatory stresses (1).
Ecologic studies in the United States (2) and elsewhere (3) have suggested an inverse correlation between estimated ultraviolet exposure and upper GI cancer rates. However, ecologic studies are principally hypothesis generating and provide the weakest evidence because of the lack of individual data on exposure and disease. In contrast, another study reported a higher risk of second primary cancers in internal organs, including the esophagus and stomach, after a first diagnosis of nonmelanoma skin cancer, but these associations seemed limited to countries with lower ultraviolet exposure and did not show specificity by cancer site (4). Another study built an index from factors that predict higher serum 25-hydroxyvitamin D (25(OH)D) concentrations (dietary and supplemental vitamin D, skin pigmentation, adiposity, geographic region of residence, and leisure-time physical activity) and found that index values that predict higher vitamin D status were associated with a statistically significant lower risk of esophageal cancer and a non-statistically-significant lower risk of stomach cancer (5). This index, however, included exposures that may affect cancer risk independent from their association with vitamin D status.
Observational studies with individual exposure metrics have produced mixed results. Case-control studies of upper GI cancer examining dietary and/or supplemental vitamin D have reported that higher vitamin D intake is associated with lower risk of esophageal squamous cell carcinoma (ESCC) (6), is associated with increased risk of gastric cancer (7), or had no association with gastric cancer (8). A prospective cohort study from China showed that higher serum 25(OH)D concentrations were associated with higher risk of ESCC but had no association with risk of gastric cancer (9). Another study in the same population showed that higher 25(OH)D concentrations were associated with higher risk of squamous dysplasia, the precursor lesion for ESCC (10).
The current study examined the association between circulating 25(OH)D concentration and upper GI cancer risk in a nested case-control study combining gastric and esophageal cancer cases and matched controls from 8 prospective cohort studies from China, Finland, and the United States as part of the Cohort Consortium Vitamin D Pooling Project of Rarer Cancers (VDPP). To maximize power, total upper GI cancer was the primary outcome.
MATERIALS AND METHODS
Study design and population
A detailed description of the cohorts and methods used in the VDPP is provided in the paper by Gallicchio et al. (11). The upper GI cancer analyses included esophageal and gastric cancer cases from the following 8 cohorts: the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study (ATBC); CLUE; the Cancer Prevention Study II (CPS-II) Nutrition Cohort; the Multiethnic Cohort Study (MEC); the New York University Women's Health Study (NYU-WHS); the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCO); the Shanghai Men's Health Study (SMHS); and the Shanghai Women's Health Study (SWHS). The number of subjects and other information for each cohort are given in Table 1.
Table 1.
Characteristics of Participants, by Cohort, in the Investigation of Upper Gastrointestinal Cancer Within the Cohort Consortium Vitamin D Pooling Project of Rarer Cancers
| Cohort | No. of Cases | No. of Controls | Median Years From Blood Collection to Cancer Diagnosis (Interquartile Range) | Median Circulating 25(OH)D, nmol/L (Interquartile Range) |
|
| Cases | Controls | ||||
| ATBC | 416 | 417 | 8.7 (5.1–13.1) | 30.8 (20.0–43.3) | 31.5 (19.6–46.8) |
| CLUE | 88 | 88 | 10.6 (5.5–16.5) | 59.3 (45.4–81.7) | 61.5 (45.4–80.9) |
| CPS-II | 40 | 40 | 1.9 (1.4–3.8) | 58.4 (46.6–71.4) | 58.2 (46.6–69.0) |
| MEC | 82 | 82 | 2.2 (1.1–3.4) | 47.8 (33.6–66.3) | 47.0 (34.5–64.8) |
| NYU-WHS | 27 | 27 | 11.8 (7.0–16.2) | 41.0 (26.3–51.3) | 38.6 (28.7–51.3) |
| PLCO | 99 | 99 | 5.5 (2.9–6.9) | 56.7 (42.4–68.3) | 55.8 (41.1–68.5) |
| SMHS | 131 | 131 | 1.7 (0.9–2.9) | 41.8 (29.6–57.5) | 39.0 (29.5–53.6) |
| SWHS | 182 | 182 | 4.6 (2.5–6.6) | 36.6 (24.3–47.5) | 35.1 (24.7–45.7) |
| Total | 1,065 | 1,066 | 5.3 (2.4–9.1) | 39.4 (26.3–56.1) | 39.3 (26.1–56.3) |
Abbreviations: ATBC, Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study; CPS-II, Cancer Prevention Study II Nutrition Cohort; MEC, Multiethnic Cohort Study; NYU-WHS, New York University Women's Health Study; 25(OH)D, 25-hydroxyvitamin D; PLCO, Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial; SMHS, Shanghai Men's Health Study; SWHS, Shanghai Women's Health Study.
Tumor location and histologic coding methods varied by study and included the International Classification of Diseases, Ninth Revision; International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; and country-specific methods. For the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, cases with epithelial tumors located in the esophagus (codes C152–159), gastric cardia (code C160), body of the stomach (codes C161–166), and overlapping and not otherwise specified locations (codes C168–169) were included. Cases were matched to controls who were alive and cancer free at the time of case diagnosis. Controls were matched within cohorts to cases on age at blood collection (±1 year), sex, race/ethnicity (Asian/black/Caucasian/other), and calendar day of blood draw (±30 days).
Of the 1,077 cases initially identified, 12 were excluded because of a diagnostic date before blood draw (n = 2), ineligible histology (n = 5), failed 25(OH)D assay (n = 1), and lack of an adequate control (n = 4), leaving 1,065 cases and 1,066 controls for analysis. Case and control numbers are uneven because 1 subject was simultaneously diagnosed with esophageal and stomach cancer and was matched to 2 different controls. In disease-stratified analyses, this subject was included for both cancer sites but only once in the total upper GI cancer analyses. Any upper GI cancer was the main outcome to maximize power, but subgroup analyses were conducted based on organ and histology, where possible, including all esophageal cancer (n = 265), ESCC (n = 142), esophageal adenocarcinoma (n = 104), all gastric cancers (n = 784), gastric cardia adenocarcinoma (n = 135), and gastric noncardia adenocarcinoma (n = 428).
Measurement of circulating 25(OH)D
Circulating (serum or plasma) 25(OH)D concentrations were assayed by using a direct, competitive chemiluminescence immunoassay using the DiaSorin LIAISON 25 OH Vitamin D TOTAL Assay (11, 12). Coefficients of variation for duplicate serum/plasma aliquots included in all laboratory sample batches were calculated by using the 2 masked standards provided by the National Institute of Standards and Technology (NIST): level 1 (∼60 nmol/L) and level 2 (∼35 nmol/L). Interbatch and intrabatch coefficients of variation for level 1 samples were 12.7% and 9.3%, respectively; interbatch and intrabatch coefficients of variation for level 2 samples were 13.6% and 11.0%, respectively. For all primary analyses, a priori categories were used based on clinically defined cutpoints: <25, 25–<37.5, 37.5–<50, 50–<75, 75–<100, and ≥100 nmol/L. The category 50–<75 nmol/L was chosen as the reference because it encompasses the mean for subjects in the National Health and Nutrition Examination Survey (13). For some subgroup analyses, alternative constructs were used, including considering the <25 nmol/L category as the referent group (to aid interpretability) and using log-transformed continuous 25(OH)D (to potentially maximize power and simplify presentation).
Statistical analyses
All statistical analyses were carried out at Information Management Services, Inc. (Silver Spring, Maryland) by using SAS software, versions 9.1.3 and 9.2 (SAS Institute, Inc., Cary, North Carolina), and meta analyses were conducted by using the R function MiMa (14). Reported P values were derived from 2-sided tests, and those <0.05 were considered statistically significant.
Conditional logistic regression models were used for the primary analyses of the association between circulating 25(OH)D and upper GI cancer risk, whereas unconditional models were used for stratified models. Estimated odds ratios and 95% confidence intervals were calculated by using the 6 clinically defined categories in models without further adjustment and in multivariate-adjusted models. Trend tests of the overall association were conducted by using a 1 degree-of-freedom test with subjects assigned a value of 1–6 based on their 25(OH)D category. Conditional and unconditional models (data not shown) produced similar results. Models using cohort- and season-specific quartiles produced results similar to those using the clinically defined cutpoints (data not shown).
Potential confounding variables assessed included cigarette smoking, alcohol drinking, educational attainment (as a proxy for socioeconomic status), body mass index, and history of gastric surgery where available. Bivariate analyses to test for independent associations between potentially confounding variables and both case status (conditional logistic regression) and circulating 25(OH)D concentrations among controls (linear regression) based on the Wald test were conducted. Variables with an independent association with both case status and circulating 25(OH)D concentration (P < 0.10), as well as those with known associations with cancer risk from previous studies, were retained; then, a parsimonious final model was built with a forward selection and backward elimination procedure, looking for changes in the 25(OH)D betas of ±10%. Body mass index was excluded as a confounder and therefore the multivariate models included cigarette smoking, alcohol drinking, education, and history of gastric surgery as categorized in Table 2. For variables for which data were missing, including history of gastric surgery (data missing for 38% of cases and 27% of controls), ethnicity, smoking, alcohol drinking, and education (missing for ≤1% of subjects), a categorical variable to account for missingness was included. Unconditional models included both the matching and additional adjustment variables.
Table 2.
Selected Characteristics of Cases and Controls in the Investigation of Upper Gastrointestinal Cancer Within the Cohort Consortium Vitamin D Pooling Project of Rarer Cancers
| Cases (N = 1,065) |
Controls (N = 1,066) |
||||||
| Characteristic | No. | % | Median (Interquartile Range) | No. | % | Median (Interquartile Range) | P Valuea |
| Age at blood draw, years | 61 (55–67) | 61 (55–66) | Matched | ||||
| Sex | |||||||
| Female | 290 | 27 | 290 | 27 | Matched | ||
| Male | 775 | 73 | 776 | 73 | |||
| Race/ethnic group | |||||||
| Caucasian | 647 | 61 | 649 | 61 | Matched | ||
| Black | 30 | 3 | 30 | 3 | |||
| Asian | 350 | 33 | 349 | 33 | |||
| Other | 35 | 3 | 36 | 3 | |||
| Missing | 3 | <1 | 2 | <1 | |||
| Cigarette smoking | |||||||
| Never | 305 | 29 | 344 | 32 | 0.0001 | ||
| Quit >15 years ago | 85 | 8 | 107 | 10 | |||
| Quit 10–15 years ago | 62 | 6 | 40 | 4 | |||
| Quit 1–<10 years ago | 39 | 4 | 28 | 3 | |||
| Current, <20 cigarettes/day | 214 | 20 | 250 | 23 | |||
| Current, ≥20 cigarettes/day | 352 | 33 | 291 | 27 | |||
| Missing | 8 | <1 | 6 | <1 | |||
| Alcohol intake, g/day | |||||||
| None | 406 | 38 | 426 | 40 | 0.13 | ||
| >0–14 | 284 | 27 | 316 | 30 | |||
| >14–28 | 120 | 11 | 120 | 11 | |||
| >28 | 153 | 14 | 122 | 11 | |||
| Missing | 102 | 10 | 82 | 8 | |||
| Education | |||||||
| Less than high school | 440 | 41 | 384 | 36 | 0.0062 | ||
| Completed high school | 157 | 15 | 164 | 15 | |||
| Vocational school | 211 | 10 | 202 | 19 | |||
| Some college | 160 | 15 | 186 | 18 | |||
| College graduate | 47 | 4 | 71 | 7 | |||
| Graduate studies | 40 | 4 | 51 | 5 | |||
| Missing | 10 | 1 | 8 | 1 | |||
| Body mass index, kg/m2 | 25.3 (22.9–28.2) | 25.3 (23.1–28.1) | 0.51 | ||||
| History of gastric surgery | |||||||
| No | 620 | 58 | 758 | 71 | 0.0003 | ||
| Yes | 41 | 4 | 16 | 2 | |||
| Missing | 404 | 38 | 292 | 27 | |||
| Season of blood draw | |||||||
| Winter | 560 | 53 | 556 | 52 | 0.55 | ||
| Summer | 505 | 47 | 510 | 48 | |||
| Serum 25(OH)D concentration, nmol/L | 39.4 (26.3–56.1) | 39.1 (25.8–56.7) | 0.90 | ||||
| Serum 25(OH)D concentration category, nmol/L | |||||||
| <25 | 241 | 22.7 | 252 | 23.6 | 0.97 | ||
| 25–<37.5 | 248 | 23.2 | 239 | 22.4 | |||
| 37.5–<50 | 224 | 21.0 | 223 | 20.9 | |||
| 50–<75 | 249 | 23.4 | 252 | 23.6 | |||
| 75–<100 | 83 | 7.8 | 77 | 7.2 | |||
| ≥100 | 20 | 1.9 | 23 | 2.2 | |||
Abbreviation: 25(OH)D, 25-hydroxyvitamin D.
Wald test from conditional logistic regression, excluding subjects with missing data.
Stratified analyses were conducted by season (summer (June–November)/winter (December–May)), sex, organ, organ and histology, smoking (never/ever), alcohol drinking (4 categories), and length of follow-up (<2 years, ≥2 years). Models stratified on alcohol drinking did not include some adjusting variables because of small cell counts. We found no differences by follow-up time (data not shown). Stratified analysis by age, body mass index, physical activity, and follow-up time were conducted by using long-transformed continuous concentrations. Seasonally adjusted 25(OH)D concentrations were created by using the residuals after regression against week of blood draw using the local polynomial regression (loess) method. These results were similar to the non-seasonally-adjusted results and are not shown in this paper.
In addition to the pooled analysis described above, a meta-analysis approach was also used (14). For each cohort separately, with 50–<75 nmol/L as the referent category, odds ratios and 95% confidence intervals for subjects in the bottom (<25 nmol/L) category and for the top 2 categories combined (≥75 nmol/L) were estimated. Pooled estimates of effect using inverse-variance-weighted random-effects models were calculated and statistical heterogeneity assessed by Q and I2 statistics.
RESULTS
Table 1 presents the number of cases and controls from each cohort, the median time from blood draw to case diagnosis, and the median (interquartile range) circulating 25(OH)D concentration by cohort. Median follow-up time ranged from 1.7 years in the Shanghai Men's Health Study to 11.8 years in the New York University Women's Health Study. The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study had the lowest median 25(OH)D concentration at 31.5 nmol/L among controls, whereas CLUE had the highest at 61.5 nmol/L. Median concentrations by cohort varied based on the sex ratios, ethnic makeup, seasons of blood collection, and other factors. The predictors of 25(OH)D concentration in the VDPP cohorts were evaluated separately (15).
Table 2 shows subject characteristics by case status. Compared with controls, upper GI cancer cases reported significantly more cigarette smoking and less education, and they were more likely to report a history of gastric surgery. Cases and controls did not differ in body mass index and were comparable regarding matching factors.
Median circulating 25(OH)D concentration did not differ significantly (P = 0.90) between upper GI cancer cases and controls—39.4 nmol/L (interquartile range: 26.3–56.1) and 39.1 nmol/L (interquartile range: 25.8–56.7), respectively (Table 2). Furthermore, the distribution of cases and controls did not differ across the 6 categories of 25(OH)D (P = 0.97).
Table 3 presents the results of primary analyses of the association between circulating 25(OH)D concentration and risk of upper GI cancer overall, by season and by sex. Multivariate adjustment for potentially confounding factors had little impact on the estimates. In the overall analysis and in all subgroupings presented here, no association between 25(OH)D concentration and risk of upper GI cancer was observed. No stratum showed statistically significant associations for individual categories or trend tests. Further subdividing into 4 strata according to sex and season also showed no significant associations (data not shown).
Table 3.
Odds Ratios and 95% Confidence Intervals for the Association Between Circulating 25(OH)D and Risk of Upper Gastrointestinal Cancer Overall and by Season or Sex Within the Cohort Consortium Vitamin D Pooling Project of Rarer Cancers
| Circulating 25(OH)D, nmol/L |
Ptrend | |||||||||||||||||||||||
| <25 |
25–<37.5 |
37.5–<50 |
50–<75a |
75–<100 |
≥100 |
|||||||||||||||||||
| No. of Cases | No. of Controls | OR | 95% CI | No. of Cases | No. of Controls | OR | 95% CI | No. of Cases | No. of Controls | OR | 95% CI | No. of Cases | No. of Controls | OR | No. of Cases | No. of Controls | OR | 95% CI | No. of Cases | No. of Controls | OR | 95% CI | ||
| All subjectsb | 241 | 252 | 248 | 239 | 224 | 223 | 249 | 252 | 83 | 77 | 20 | 23 | ||||||||||||
| Crude | 0.96 | 0.72, 1.28 | 1.04 | 0.80, 1.37 | 1.02 | 0.79, 1.33 | 1.0 | 1.09 | 0.76, 1.57 | 0.88 | 0.45, 1.70 | 0.79 | ||||||||||||
| Multivariate adjusted | 0.90 | 0.65, 1.24 | 1.03 | 0.76, 1.39 | 0.92 | 0.69, 1.23 | 1.0 | 1.17 | 0.79, 1.75 | 0.81 | 0.39, 1.69 | 0.54 | ||||||||||||
| All subjects, winterc | 183 | 202 | 161 | 136 | 105 | 110 | 83 | 80 | 24 | 25 | 4 | 3 | ||||||||||||
| Crude | 0.89 | 0.60, 1.32 | 1.16 | 0.78, 1.74 | 0.93 | 0.62, 1.42 | 1.0 | 0.96 | 0.50, 1.84 | 1.20 | 0.24, 5.94 | 0.71 | ||||||||||||
| Multivariate adjusted | 0.87 | 0.57, 1.31 | 1.13 | 0.74, 1.72 | 0.89 | 0.58, 1.38 | 1.0 | 0.82 | 0.41, 1.65 | 0.97 | 0.17, 5.43 | 0.81 | ||||||||||||
| All subjects, summerc | 58 | 50 | 87 | 103 | 119 | 113 | 166 | 172 | 59 | 52 | 16 | 20 | ||||||||||||
| Crude | 1.18 | 0.75, 1.87 | 0.86 | 0.60, 1.25 | 1.07 | 0.76, 1.51 | 1.0 | 1.17 | 0.76, 1.81 | 0.83 | 0.41, 1.69 | 1.00 | ||||||||||||
| Multivariate adjusted | 0.90 | 0.55, 1.46 | 0.79 | 0.53, 1.17 | 0.98 | 0.68, 1.40 | 1.0 | 1.30 | 0.82, 2.06 | 0.83 | 0.40, 1.74 | 0.23 | ||||||||||||
| Menb | 177 | 190 | 176 | 152 | 149 | 155 | 190 | 193 | 63 | 65 | 20 | 21 | ||||||||||||
| Crude | 0.95 | 0.68, 1.33 | 1.19 | 0.86, 1.63 | 0.99 | 0.72, 1.34 | 1.0 | 0.97 | 0.64, 1.47 | 0.95 | 0.48, 1.89 | 0.85 | ||||||||||||
| Multivariate adjusted | 0.89 | 0.61, 1.31 | 1.23 | 0.85, 1.76 | 0.87 | 0.62, 1.24 | 1.0 | 1.03 | 0.65, 1.64 | 0.88 | 0.41, 1.89 | 0.97 | ||||||||||||
| Womenb | 64 | 62 | 72 | 87 | 75 | 68 | 59 | 59 | 20 | 12 | 0 | 2 | ||||||||||||
| Crude | 0.98 | 0.55, 1.73 | 0.79 | 0.46, 1.34 | 1.10 | 0.66, 1.83 | 1.0 | 1.83 | 0.81, 4.14 | 0.39 | ||||||||||||||
| Multivariate adjusted | 0.88 | 0.47, 1.65 | 0.78 | 0.44, 1.38 | 0.96 | 0.56, 1.67 | 1.0 | 1.79 | 0.74, 4.33 | 0.34 | ||||||||||||||
Abbreviations: CI, confidence interval; 25(OH)D, 25-hydroxyvitamin D; OR, odds ratio.
Reference category.
Conditional logistic regression models were matched on cohort, race, sex, and date of blood draw without or with further adjustment for alcohol drinking, smoking, education, and history of gastric surgery.
Unconditional logistic regression models were adjusted for the matching factors cohort, race, sex, and date of blood draw without or with further adjustment for alcohol drinking, smoking, education, and history of gastric surgery.
Subgroup analyses by organ site and histology are shown in Table 4. No associations were observed for total esophageal cancer or when subdivided into the 2 histologic types ESCC and esophageal adenocarcinoma. Total gastric cancer showed no association, but when divided into the 2 primary gastric subsites, cardia and noncardia stomach, an increased risk of gastric noncardia cancer with higher circulating 25(OH)D concentration was observed. Compared with the reference group (50–<75 nmol/L), those in the higher category (75–<100 nmol/L) were at statistically significantly higher risk (odds ratio (OR) = 2.00, 95% confidence interval (CI): 1.03, 3.91. The test for trend across categories was P = 0.083.
Table 4.
Odds Ratios and 95% Confidence Intervalsa for the Association Between Circulating 25(OH)D and Risk of Upper Gastrointestinal Cancer by Organ, Organ Subsite, Histology, and Smoking Status Within the Cohort Consortium Vitamin D Pooling Project of Rarer Cancers
| Circulating 25(OH)D, nmol/L |
||||||||||||||||||||||||
| <25 |
25–<37.5 |
37.5–<50 |
50–<75b |
75–<100 |
≥100 |
Ptrend | ||||||||||||||||||
| No. of Cases | No. of Controls | OR | 95% CI | No. of Cases | No. of Controls | OR | 95% CI | No. of Cases | No. of Controls | OR | 95% CI | No. of Cases | No. of Controls | OR | No. of Cases | No. of Controls | OR | 95% CI | No. of Cases | No. of Controls | OR | 95% CI | ||
| Esophageal, all | 54 | 44 | 42 | 51 | 56 | 53 | 76 | 79 | 26 | 28 | 11 | 9 | ||||||||||||
| Multivariate adjusted | 1.07 | 0.55, 2.10 | 0.70 | 0.38, 1.30 | 1.04 | 0.60, 1.80 | 1.0 | 0.98 | 0.48, 1.98 | 1.06 | 0.37, 3.05 | 0.80 | ||||||||||||
| Esophageal, ESCC | 44 | 32 | 24 | 37 | 33 | 29 | 32 | 33 | 7 | 8 | 2 | 3 | ||||||||||||
| Multivariate adjusted | 1.38 | 0.53, 3.57 | 0.49 | 0.20, 1.22 | 1.16 | 0.50, 2.66 | 1.0 | 0.96 | 0.23, 3.92 | 0.68 | 0.076, 6.02 | 0.77 | ||||||||||||
| Esophageal, EADC | 8 | 10 | 15 | 11 | 19 | 20 | 36 | 38 | 17 | 18 | 9 | 6 | ||||||||||||
| Multivariate adjusted | 0.61 | 0.47, 3.96 | 1.36 | 0.47, 3.96 | 0.95 | 0.39, 2.28 | 1.0 | 1.10 | 0.43, 2.83 | 1.17 | 0.30, 4.45 | 0.70 | ||||||||||||
| Gastric, all | 183 | 202 | 201 | 187 | 164 | 167 | 171 | 165 | 56 | 49 | 9 | 14 | ||||||||||||
| Multivariate adjusted | 0.77 | 0.55, 1.08 | 0.99 | 0.71, 1.36 | 0.88 | 0.64, 1.22 | 1.0 | 1.11 | 0.70, 1.77 | 0.65 | 0.26, 1.62 | 0.25 | ||||||||||||
| Gastric, cardia | 34 | 40 | 29 | 33 | 29 | 23 | 32 | 25 | 9 | 9 | 2 | 5 | ||||||||||||
| Multivariate adjusted | 0.64 | 0.26, 1.62 | 0.70 | 0.29, 1.71 | 1.12 | 0.46, 2.71 | 1.0 | 0.65 | 0.18, 2.32 | 0.12 | 0.011, 1.25 | 0.88 | ||||||||||||
| Gastric, noncardia | 103 | 115 | 115 | 116 | 99 | 96 | 86 | 87 | 35 | 19 | 1 | 6 | ||||||||||||
| Multivariate adjusted | 0.74 | 0.47, 1.17 | 0.87 | 0.56, 1.35 | 0.91 | 0.59, 1.41 | 1.0 | 2.00 | 1.03, 3.91 | 0.10 | 0.01, 0.99 | 0.083 | ||||||||||||
| Never smokerc | 51 | 58 | 65 | 89 | 69 | 88 | 86 | 79 | 27 | 25 | 7 | 5 | ||||||||||||
| Multivariate adjusted | 0.55 | 0.31, 0.96 | 0.47 | 0.28, 0.78 | 0.56 | 0.35, 0.90 | 1.0 | 1.24 | 0.63, 2.44 | 1.47 | 0.41, 5.30 | 0.004 | ||||||||||||
| Ever smokerc | 189 | 194 | 183 | 147 | 151 | 134 | 162 | 171 | 54 | 52 | 13 | 18 | ||||||||||||
| Multivariate adjusted | 1.02 | 0.72, 1.45 | 1.38 | 0.98, 1.95 | 1.20 | 0.85, 1.69 | 1.0 | 1.07 | 0.67, 1.69 | 0.68 | 0.30, 1.49 | 0.49 | ||||||||||||
| Nondrinkerd | 93 | 107 | 98 | 117 | 85 | 98 | 98 | 82 | 25 | 16 | 5 | 5 | ||||||||||||
| Multivariate adjusted | 0.75 | 0.48, 1.16 | 0.70 | 0.46, 1.07 | 0.72 | 0.47, 1.09 | 1.0 | 1.32 | 0.66, 2.66 | 0.88 | 0.24, 3.16 | 0.081 | ||||||||||||
| Drinks >0–14 g/day of alcoholc | 66 | 75 | 57 | 65 | 55 | 59 | 68 | 78 | 33 | 30 | 5 | 8 | ||||||||||||
| Multivariate adjusted | 0.94 | 0.57, 1.53 | 0.95 | 0.57, 1.57 | 1.02 | 0.62, 1.68 | 1.0 | 1.19 | 0.65, 2.17 | 0.72 | 0.22, 2.34 | 0.64 | ||||||||||||
| Drinks >14 g/day of alcohold | 65 | 57 | 76 | 45 | 60 | 51 | 53 | 64 | 14 | 14 | 5 | 8 | ||||||||||||
| Multivariate adjusted | 1.42 | 0.83, 2.45 | 2.06 | 1.20, 3.54 | 1.39 | 0.81, 2.38 | 1.0 | 0.96 | 0.43, 2.14 | 0.69 | 0.21, 2.27 | 0.034 | ||||||||||||
| Missing data for alcohol drinkingd | 17 | 13 | 17 | 12 | 23 | 14 | 29 | 27 | 10 | 14 | 5 | 2 | ||||||||||||
| Multivariate adjusted | 1.30 | 0.48, 3.56 | 1.36 | 0.49, 3.79 | 1.54 | 0.64, 3.70 | 1.0 | 0.65 | 0.24, 1.74 | 2.37 | 0.42, 13.37 | 0.55 | ||||||||||||
Abbreviations: CI, confidence interval; EADC, esophageal adenocarcinoma; ESCC, esophageal squamous cell carcinoma; 25(OH)D, 25-hydroxyvitamin D; OR, odds ratio.
Unconditional logistic regression models were adjusted for the matching factors cohort, race, sex, and date of blood draw, with further adjustment for alcohol drinking, smoking, education, and history of gastric surgery. The adjustments for models stratified on alcohol drinking are given in footnote c.
Reference category.
The test for interaction with smoking was statistically significant, P = 0.015.
Unconditional logistic regression models were adjusted for the matching factors race, sex, and date of blood draw, without further adjustment. The test for interaction with drinking was statistically significant, P = 0.0025.
Among never smokers, those in the lowest category of 25(OH)D concentrations were at lower risk of upper GI cancer compared with the reference group (OR = 0.55, 95% CI: 0.31, 0.96), with a statistically significant test for trend (P = 0.004). No statistically significant associations were observed among ever smokers (Table 4).
In models stratified on alcohol drinking, some differences were found (Table 4). Subjects consuming more than 14 g of alcohol a day showed higher risk of upper GI cancer at lower vitamin D concentrations, whereas an opposite trend appeared to be present among those who did not drink alcohol.
Associations varied by race (Table 5), but sufficient numbers to examine models separately were available for only Asians and Caucasians. Among Asians, a statistically significant decreased risk of upper GI cancer was observed with lower concentrations, with a significant trend across categories of 25(OH)D concentration (P = 0.003). For example, subjects in the lowest category (<25 nmol/L) had a 47% (95% CI: 9, 69) lower risk of upper GI cancer than those in the referent group (50–<75 nmol/L). To simplify interpretation, models were also fit by using the lowest category as the referent. The odds ratio estimates for increasing 25(OH)D categories were 1.0 (reference), 0.94 (95% CI: 0.60, 1.48), 0.96 (95% CI: 0.59, 1.56), 1.88 (95% CI: 1.10, 3.22), 3.56 (95% CI: 1.39, 9.14), and 1.85 (95% CI: 0.44, 7.75). Although sample sizes became sparse, further stratification by season or by sex found significant trend tests in all subgroups of Asians except women alone. Among Caucasians, no statistically significant associations were observed between circulating 25(OH)D and risk of upper GI cancer overall or in groups defined by sex or season. Stratified analyses among the overall population by age, body mass index, physical activity, and follow-up time showed no evidence that the associations differed by these strata (Table 6).
Table 5.
Odds Ratios and 95% Confidence Intervalsa for the Association Between Circulating 25(OH)D and Risk of Upper Gastrointestinal Cancer by Ethnic Group, by Season and Sex Within Ethnic Group, Within the Cohort Consortium Vitamin D Pooling Project of Rarer Cancers
| Circulating 25(OH)D, nmol/L |
||||||||||||||||||||||||
| <25 |
25–<37.5 |
37.5–<50 |
50–<75b |
75–<100 |
≥100 |
Ptrend | ||||||||||||||||||
| No. of Cases | No. of Controls | OR | 95% CI | No. of Cases | No. of Controls | OR | 95% CI | No. of Cases | No. of Controls | OR | 95% CI | No. of Cases | No. of Controls | OR | No. of Cases | No. of Controls | OR | 95% CI | No. of Cases | No. of Controls | OR | 95% CI | ||
| Asians, all | 67 | 71 | 92 | 111 | 73 | 88 | 88 | 63 | 24 | 11 | 6 | 5 | ||||||||||||
| Multivariate adjusted | 0.53 | 0.31, 0.91 | 0.50 | 0.31, 0.80 | 0.51 | 0.32, 0.82 | 1.0 | 1.89 | 0.78, 4.60 | 0.99 | 0.25, 3.91 | 0.003 | ||||||||||||
| Asians, winter | 52 | 55 | 60 | 63 | 31 | 37 | 25 | 12 | 7 | 5 | 1 | 0 | ||||||||||||
| Multivariate adjusted | 0.29 | 0.11, 0.73 | 0.29 | 0.12, 0.72 | 0.27 | 0.10, 0.69 | 1.0 | 0.76 | 0.13, 4.30 | 0.049 | ||||||||||||||
| Asians, summer | 15 | 16 | 32 | 48 | 42 | 51 | 63 | 51 | 17 | 6 | 5 | 5 | ||||||||||||
| Multivariate adjusted | 0.79 | 0.34, 1.83 | 0.57 | 0.31, 1.05 | 0.67 | 0.37, 1.21 | 1.0 | 3.24 | 1.03, 10.17 | 1.06 | 0.23, 4.91 | 0.027 | ||||||||||||
| Asians, men | 19 | 24 | 41 | 45 | 26 | 42 | 51 | 37 | 18 | 9 | 6 | 4 | ||||||||||||
| Multivariate adjusted | 0.46 | 0.20, 1.08 | 0.57 | 0.29, 1.12 | 0.38 | 0.19, 0.78 | 1.0 | 2.05 | 0.70, 6.01 | 1.28 | 0.28, 5.91 | 0.014 | ||||||||||||
| Asians, women | 48 | 47 | 51 | 66 | 47 | 46 | 37 | 26 | 6 | 2 | 0 | 1 | ||||||||||||
| Multivariate adjusted | 0.65 | 0.31, 1.34 | 0.51 | 0.26, 1.01 | 0.68 | 0.34, 1.33 | 1.0 | 2.01 | 0.35, 11.48 | 0.16 | ||||||||||||||
| Caucasians, all | 155 | 167 | 140 | 115 | 134 | 115 | 150 | 172 | 54 | 62 | 14 | 18 | ||||||||||||
| Multivariate adjusted | 0.98 | 0.67, 1.44 | 1.43 | 0.98, 2.08 | 1.28 | 0.89, 1.84 | 1.0 | 1.07 | 0.68, 1.69 | 0.81 | 0.37, 1.79 | 0.69 | ||||||||||||
| Caucasians, winter | 119 | 140 | 93 | 67 | 71 | 65 | 51 | 58 | 16 | 16 | 3 | 3 | ||||||||||||
| Multivariate adjusted | 0.98 | 0.58, 1.65 | 1.73 | 1.00, 2.97 | 1.25 | 0.72, 2.17 | 1.0 | 1.00 | 0.41, 2.40 | 0.77 | 0.11, 5.28 | 0.98 | ||||||||||||
| Caucasians, summer | 36 | 27 | 47 | 48 | 63 | 50 | 99 | 114 | 38 | 46 | 11 | 15 | ||||||||||||
| Multivariate adjusted | 1.06 | 0.54, 2.10 | 1.09 | 0.63, 1.90 | 1.37 | 0.83, 2.27 | 1.0 | 1.07 | 0.62, 1.85 | 0.78 | 0.32, 1.91 | 0.57 | ||||||||||||
| Caucasians, men | 149 | 159 | 127 | 102 | 114 | 101 | 132 | 146 | 42 | 54 | 14 | 17 | ||||||||||||
| Multivariate adjusted | 0.94 | 0.63, 1.40 | 1.40 | 0.93, 2.10 | 1.20 | 0.81, 1.77 | 1.0 | 0.91 | 0.55, 1.51 | 0.85 | 0.37, 1.90 | 0.73 | ||||||||||||
| Caucasians, women | 6 | 8 | 13 | 13 | 20 | 14 | 18 | 26 | 12 | 8 | 0 | 1 | ||||||||||||
| Multivariate adjusted | 0.80 | 0.17, 3.71 | 1.71 | 0.50, 5.92 | 1.42 | 0.46, 4.38 | 1.0 | 2.95 | 0.76, 11.46 | 0.73 | ||||||||||||||
Abbreviations: CI, confidence interval; 25(OH)D, 25-hydroxyvitamin D; OR, odds ratio.
Unconditional logistic regression models were adjusted for the matching factors cohort, race, sex, and date of blood draw, with further adjustment for alcohol drinking, smoking, education, and history of gastric surgery, as appropriate within strata. The test for interaction with ethnicity was statistically significant, P = 0.0021.
Reference category.
Table 6.
Odds Ratios and 95% Confidence Intervals for the Association Between Circulating 25(OH)Da and Risk of Upper Gastrointestinal Cancer by Selected Strata Within the Cohort Consortium Vitamin D Pooling Project of Rarer Cancers
| OR | 95% CI | |
| Age at blood draw, years | ||
| ≤61 | 1.07 | 0.84, 1.36 |
| >61 | 1.06 | 0.80, 1.39 |
| Body mass index, kg/m2 | ||
| <25 | 1.00 | 0.77, 1.30 |
| 25–<30 | 1.28 | 0.94, 1.74 |
| ≥30 | 0.82 | 0.47, 1.43 |
| Physical activity | ||
| Sedentary | 0.97 | 0.73, 1.30 |
| Light | 0.98 | 0.69, 1.38 |
| Moderate | 1.60 | 0.85, 3.00 |
| Vigorous | 1.10 | 0.63, 1.91 |
| Follow-up time, years | ||
| <2 | 1.19 | 0.83, 1.71 |
| ≥2 | 1.04 | 0.84, 1.28 |
Abbreviations: CI, confidence interval; 25(OH)D, 25-hydroxyvitamin D; OR, odds ratio.
Modeled as 1 log unit of 25(OH)D in unconditional logistic regression models adjusted for the matching factors cohort, race, sex, and date of blood draw, with further adjustment for alcohol drinking, smoking, education, and history of gastric surgery, as appropriate within strata.
Models for each of the 4 specific cancer outcomes that were simultaneously stratified on ethnicity, smoking, or alcohol drinking had small numbers and produced most risk estimates with wide confidence intervals (data not shown). These results were similar to those presented in that low vitamin D concentrations were associated with lower risk of noncardia gastric cancer in Asians and lower risk of esophageal adenocarcinoma in whites. In contrast, risk of ESCC was nonsignificantly higher with lower concentrations in whites and Asians.
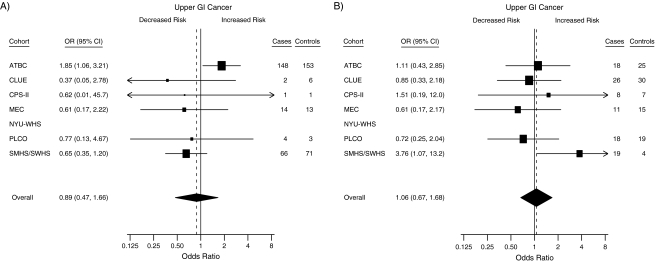
Finally, the association between circulating 25(OH)D concentration and upper GI cancer risk was examined by using a meta-analysis framework. Figure 1 shows the odds ratio in each cohort separately comparing those in the <25 nmol/L group with the referent group of 50–<75 nmol/L and those in the ≥75 nmol/L group compared with the same group. For subjects with circulating concentrations of <25 nmol/L, most estimates were below unity, and the summary odds ratio across cohorts was 0.89 (95% CI: 0.47, 1.66), although there was some heterogeneity in these estimates (I2 = 39%; P = 0.14). The estimate for the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study cohort appeared to differ from the rest in showing significantly increased risk (OR = 1.85, 95% CI: 1.06, 3.21). For subjects with circulating concentrations of ≥75 nmol/L, risk estimates centered on unity with an odds ratio across cohorts of 1.06 (95% CI: 0.67, 1.68) and little heterogeneity (I2 = 9%; P = 0.36).
Figure 1.
Forest plots for the meta-analysis of the association between circulating 25-hydroxyvitamin D (25(OH)D) and the risk of esophageal and gastric cancer within the Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Risk estimates, by cohort, for subjects with circulating 25(OH)D concentrations of A) <25 nmol/L and B) ≥75 nmol/L compared with the referent group (50–<75 nmol/L). Odds ratios (ORs) and 95% confidence intervals (CIs) were derived from conditional logistic regression models. The boxes show the odds ratios, the bars show the 95% confidence intervals, and the size of each box is inversely proportional to the variance of the log odds ratio estimate in each cohort. The overall estimates (diamonds) were derived from a meta-analysis using random-effects modeling. No estimates are given for the NYU-WHS because of small numbers in the exposed group. For the <25 nmol/L comparison, I2 was 39%; for the ≥75 nmol/L comparison, I2 was 9%. ATBC, Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study; CPS-II, Cancer Prevention Study II Nutrition Cohort; GI, gastrointestinal; MEC, Multiethnic Cohort Study; NYU-WHS, New York University Women's Health Study; PLCO, Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial; SMHS/SWHS, Shanghai Men's Health Study/Shanghai Women's Health Study.
DISCUSSION
This study examined the association between circulating 25(OH)D concentrations and upper GI cancer risk in a combined analysis nested in 8 prospective cohort studies. Overall, no association between circulating 25(OH)D concentration and risk of upper GI cancers was observed. Surprisingly, in models stratified by race/ethnic group or smoking, there was evidence of a protective association with lower vitamin D status and cancer risk. In models stratified on alcohol drinking, 2 opposing trends were evident. For subjects reporting no alcohol consumption, having a lower concentration of circulating vitamin D was associated with lower cancer risk; for those reporting consumption of more than 14 g of alcohol a day, lower concentrations were associated with higher risk of cancer. Among Asians, most of whom participated in the 2 Shanghai cohorts of Han Chinese, and among never smokers, the risk of cancer was statistically significantly lower for subjects with circulating concentrations of <50 nmol/L compared with those with higher concentrations. Specifically, compared with a circulating 25(OH)D concentration of 50–75 nmol/L, a range that encompasses the mean for subjects in the National Health and Nutrition Examination Survey, the odds ratio for Asian subjects with a circulating concentration of <25 nmol/L was 0.53 (95% CI: 0.31, 0.91).
Similarly, for never smokers, the same contrast produced an odds ratio of 0.55 (95% CI: 0.31, 0.96). The estimate in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study cohort, whose members were all active smokers, appeared to differ from that in the other cohorts, showing that subjects in the lowest 25(OH)D category had higher risk of upper GI cancer (OR = 1.85, 95% CI: 1.06, 3.21). This finding is consistent with the possibility that smoking status may modify the association of vitamin D with upper GI cancer risk. All these stratified estimates should be interpreted with caution because these stratifications also altered the primary outcomes examined (e.g., more ESCC among the alcohol drinkers), and it altered the relative representation of different cohorts (e.g., all Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study subjects were among the ever smokers). However, these subgroup analyses strongly suggest that each disease subtype should be examined separately in large studies to clarify these potential interactions.
To our knowledge, only 2 previous studies have tested the association between circulating vitamin D status and risk of upper GI cancer, and both were conducted in China and examined Han Chinese (9, 10). In a prospective study, a serum 25(OH)D concentration of <20 nmol/L compared with >48 nmol/L conveyed a significantly reduced risk of 0.56 for ESCC in men but showed no association among women. In contrast, there was no evidence of association with risk of gastric cancer in either sex in that study. A subsequent cross-sectional study in the same population showed lower risk of esophageal squamous dysplasia, the preneoplastic lesion for ESCC, with lower serum 25(OH)D status. The results of the present study among Asian participants, most of whom were Han Chinese, were similar regarding the direction of association. Our sample size among Asians was too small to test for distinct associations by cancer type, but the adverse association was apparent in both sexes, a finding that differs from the previous report (9).
Studies of other cancer sites in the VDPP have also observed an association between higher vitamin D status and increased cancer risk. As in a previous study of the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study cohort alone (16), higher vitamin D status was associated with increased risk of pancreatic cancer. Here, a 2-fold increase in risk was seen for subjects with a 25(OH)D concentration of >100 nmol/L (17). In addition, the analysis of non-Hodgkin lymphoma suggested an association of increased risk with higher vitamin D status in women (18).
As in all observational epidemiologic studies, the results of this analysis should be interpreted with caution because unmeasured or poorly measured confounders could obscure the true association. Furthermore, the significant differences we observed were apparent only in subgroups. However, the magnitude of the estimated risks in these subgroups and the observed dose-response associations were such that any unmeasured factor would need to have a strong association with upper GI cancer risk and also be well correlated with vitamin D status to confound the associations observed.
One possible source of confounding not included in our pooled analysis study was occupation. Some jobs that convey lower socioeconomic status, which may increase the risk of upper GI cancers, may also entail more sun exposure. This potential confounding by occupation was explored in the Shanghai cohorts (data not shown), but these analyses did not suggest that occupational differences would explain the adverse association among Asians. Other potential confounders were considered in the analyses. Among Asian controls in the VDPP, alcohol consumption and vitamin D intake (primarily from fish) was associated with higher circulating 25(OH)D, whereas current smoking was associated with significantly lower circulating 25(OH)D (15). Because the models were adjusted for alcohol consumption and cigarette smoking, and because smoking was correlated with lower 25(OH)D concentration, confounding by smoking is unlikely to explain the association among Asians.
Several biologic rationales have been postulated for possible adverse associations between higher vitamin D status and increased cancer risk, including induction of phase I metabolizing enzymes under certain conditions (19), which may be relevant in some populations. Second, the vitamin D pathway can have both proliferative and antiproliferative effects on preneoplastic lesions in an organ-specific manner. In cells molecularly similar to esophageal squamous dysplasia in their ratio of E-cadherin to osteopontin, vitamin D may stimulate cell proliferation; in cells molecularly similar to colon polyps in their ratio of E-cadherin to osteopontin, vitamin D appears to be antiproliferative (20). However, these hypotheses remain speculative. Alternatively, vitamin D status may change with occult cancer such that reverse causation contributes to the apparent adverse association. Future analyses of these cohorts are warranted because the relatively short median follow-up in the Shanghai Men's Health Study, a major contributor of the Asian subjects in the analysis, leaves open the possibility that occult cancers influenced vitamin D concentrations.
This study has several strengths. The combination of multiple populations from diverse geographic locations provided a wide distribution of exposure to circulating 25(OH)D concentrations. Using 8 cohorts supplied a relatively large sample size for these cancers in a prospective study. Furthermore, all samples were measured by using the same methods in a single facility. Weaknesses of the study include combining cancers with some disparate risk factors under a single outcome of upper GI cancer, although analyses were also conducted by cancer type. In addition, data on several potential confounding risk factors, including Helicobacter pylori status for gastric cancer and history of gastroesophageal reflux disease for esophageal adenocarcinomas, were not available. However, no data suggest that these factors are related to vitamin D status and could confound results.
In this combined analysis of 8 prospective cohorts, no overall association between circulating 25(OH)D concentration and risk of upper GI cancers was observed. In subgroup analyses, an adverse association between higher vitamin D status and upper GI cancer risk for Asians and for never smokers, and opposing trends in subgroups defined by alcohol consumption, suggested that the association may differ by major risk factors for upper GI cancer or among individuals with different risk-factor profiles for upper GI cancer. In summary, these results do not support the hypothesis that interventions aimed at increasing vitamin D status would lead to lower risk of these highly fatal cancers.
Acknowledgments
Author affiliations: Department of Chronic Disease Prevention, National Institute for Health and Welfare, Helsinki, Finland (Jarmo Virtamo); Department of Environmental Medicine, New York University School of Medicine, New York, New York (Alan A. Arslan, Yu Chen, Richard B. Hayes); Department of Obstetrics and Gynecology, New York University School of Medicine, New York, New York (Alan A. Arslan); Department of Medicine, New York University School of Medicine, New York, New York (Yu Chen); New York University Cancer Institute, New York, New York (Alan A. Arslan, Yu Chen, Richard B. Hayes); Epidemiology Research Program, American Cancer Society, Atlanta, Georgia (Peter T. Campbell, Marjorie L. McCullough); Department of Epidemiology, Shanghai Cancer Institute, Shanghai, People's Republic of China (Yu-Tang Gao, Yong-Bing Xiang); Department of Preventive Medicine, University of Alabama at Birmingham, Birmingham, Alabama (James M. Shikany); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, Maryland (Christian C. Abnet, Demetrius Albanes, Wong-Ho Chow, Mark P. Purdue, Kai Yu, Stephanie J. Weinstein); Epidemiology Program, Cancer Research Center of Hawaii, University of Hawaii, Honolulu, Hawaii (Laurence N. Kolonel, Loïc Le Marchand, Abraham M. Y. Nomura); Heartland Assays, Inc., Ames, Iowa (Ronald L. Horst); Information Management Services, Inc., Silver Spring, Maryland (David S. Campbell, Kirk Snyder); Department of Epidemiology, The Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland (Kathy J. Helzlsouer); The Prevention and Research Center, The Weinberg Center for Women's Health and Medicine, Mercy Medical Center, Baltimore, Maryland (Kathy J. Helzlsouer); and Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, Tennessee (Xiao-Ou Shu, Wei Zheng).
This work was supported by the Extramural Research Program of the National Institutes of Health, Division of Cancer Control and Population Sciences, National Cancer Institute (NCI) (Bethesda, Maryland) and the Intramural Research Program of the National Institutes of Health, Division of Cancer Epidemiology and Genetics, NCI. The New York University Women's Health Study was supported by the NCI (grant R01 CA098661). The Multiethnic Cohort Study was supported by the NCI (grants R37 CA54281, P01 CA33619, R01 CA063464, and N01-PC35137). The Shanghai Men's Health Study was supported by the NCI (grant R01 CA82729). The Shanghai Women's Health Study was supported by the NCI (grants R37 CA70867 and N02-CP-11010-66). The Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial was supported by contracts from the NCI to the University of Colorado, Denver, Colorado (grant N01-CN-25514); Georgetown University, Washington, DC (grant N01-CN-25522); the Pacific Health Research Institute, Honolulu, Hawaii (grant N01-CN-25515); the Henry Ford Health System, Detroit, Michigan (grant N01-CN-25512); the University of Minnesota, Minneapolis, Minnesota (grant N01-CN-25513); Washington University, St. Louis, Missouri (grant NO1-CN-25516); the University of Pittsburgh, Pittsburgh, Pennsylvania (grant N01-CN-25511); the University of Utah, Salt Lake City, Utah (grant N01-CN-25524); the Marshfield Clinic Research Foundation, Marshfield, Wisconsin (grant N01-CN-25518); the University of Alabama, Birmingham, Alabama (grant NO1-CN-75022); Westat, Inc., Rockville, Maryland (grant N01-CN-25476); and the University of California, Los Angeles, Los Angeles, California (grant NO1-CN-25404). The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study was supported by funding provided by the Intramural Research Program of the NCI and US Public Health Service contracts (N01-CN-45165, N01-RC-45035, N01-RC-37004). CLUE was supported by the National Institute on Aging (grant U01 AG018033) and the NCI (grants R01 CA105069, K07 CA73790). The participation of CLUE investigators was also supported by an NCI contract awarded to Mercy Medical Center through the University of Hawaii (Honolulu, Hawaii). The Cancer Prevention Study II Nutrition Cohort was supported by the American Cancer Society (Atlanta, Georgia).
The authors thank Dr. Karen Phinney of the National Institute of Standards and Technology for providing the SRM 972 Vitamin D in Human Serum used in this work.
Members of the VDPP Upper GI Writing Committee: Christian C. Abnet, Yu Chen, Wong-Ho Chow, Yu-Tang Gao, Kathy J. Helzlsouer, Loïc Le Marchand, Marjorie L. McCullough, James M. Shikany, and Xiao-Ou Shu.
This report is based at least in part on information provided by the Maryland Cancer Registry, Maryland Department of Health and Mental Hygiene.
Dr. Ronald L. Horst is the President and Chief Executive Officer of Heartland Assays, Inc.
Glossary
Abbreviations
- CI
confidence interval
- ESCC
esophageal squamous cell carcinoma
- GI
gastrointestinal
- 25(OH)D
25-hydroxyvitamin D
- OR
odds ratio
- VDPP
Cohort Consortium Vitamin D Pooling Project of Rarer Cancers
References
- 1.Fleet JC. Molecular actions of vitamin D contributing to cancer prevention. Mol Aspects Med. 2008;29(6):388–396. doi: 10.1016/j.mam.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grant WB. An estimate of premature cancer mortality in the U.S. due to inadequate doses of solar ultraviolet-B radiation. Cancer. 2002;94(6):1867–1875. doi: 10.1002/cncr.10427. [DOI] [PubMed] [Google Scholar]
- 3.Grant WB. Does solar ultraviolet irradiation affect cancer mortality rates in China? Asian Pac J Cancer Prev. 2007;8(2):236–242. [PubMed] [Google Scholar]
- 4.Tuohimaa P, Pukkala E, Scélo G, et al. Does solar exposure, as indicated by the non-melanoma skin cancers, protect from solid cancers: vitamin D as a possible explanation. Eur J Cancer. 2007;43(11):1701–1712. doi: 10.1016/j.ejca.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 5.Giovannucci E, Liu Y, Rimm EB, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98(7):451–459. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 6.Launoy G, Milan C, Day NE, et al. Diet and squamous-cell cancer of the oesophagus: a French multicentre case-control study. Int J Cancer. 1998;76(1):7–12. doi: 10.1002/(sici)1097-0215(19980330)76:1<7::aid-ijc2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 7.La Vecchia C, Ferraroni M, D'Avanzo B, et al. Selected micronutrient intake and the risk of gastric cancer. Cancer Epidemiol Biomarkers Prev. 1994;3(5):393–398. [PubMed] [Google Scholar]
- 8.Pelucchi C, Tramacere I, Bertuccio P, et al. Dietary intake of selected micronutrients and gastric cancer risk: an Italian case-control study. Ann Oncol. 2009;20(1):160–165. doi: 10.1093/annonc/mdn536. [DOI] [PubMed] [Google Scholar]
- 9.Chen W, Dawsey SM, Qiao YL, et al. Prospective study of serum 25(OH)-vitamin D concentration and risk of oesophageal and gastric cancers. Br J Cancer. 2007;97(1):123–128. doi: 10.1038/sj.bjc.6603834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abnet CC, Chen W, Dawsey SM, et al. Serum 25(OH)-vitamin D concentration and risk of esophageal squamous dysplasia. Cancer Epidemiol Biomarkers Prev. 2007;16(9):1889–1893. doi: 10.1158/1055-9965.EPI-07-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallicchio L, Helzlsouer KJ, Chow WH, et al. Circulating 25-hydroxyvitamin D and the risk of rarer cancers: design and methods of the Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol. 2010;172(1):10–20. doi: 10.1093/aje/kwq116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wagner D, Hanwell HE, Vieth R. An evaluation of automated methods for measurement of serum 25-hydroxyvitamin D. Clin Biochem. 2009;42(15):1549–1556. doi: 10.1016/j.clinbiochem.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 13.Looker AC, Pfeiffer CM, Lacher DA, et al. Serum 25-hydroxyvitamin D status of the US population: 1988–1994 compared with 2000–2004. Am J Clin Nutr. 2008;88(6):1519–1527. doi: 10.3945/ajcn.2008.26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viechtbauer W. MiMa: an S-Plus/R function to fit meta-analytic mixed-, random-, and fixed-effects models [computer software and manual] 2006 ( http://www.wvbauer.com/downloads.html). (Accessed February 6, 2009) [Google Scholar]
- 15.McCullough ML, Weinstein SJ, Freedman DM, et al. Correlates of circulating 25-hydroxyvitamin D: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol. 2010;172(1):21–35. doi: 10.1093/aje/kwq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stolzenberg-Solomon RZ, Vieth R, Azad A, et al. A prospective nested case-control study of vitamin D status and pancreatic cancer risk in male smokers. Cancer Res. 2006;66(20):10213–10219. doi: 10.1158/0008-5472.CAN-06-1876. [DOI] [PubMed] [Google Scholar]
- 17.Stolzenberg-Solomon RZ, Jacobs EJ, Arslan AA, et al. Circulating 25-hydroxyvitamin D and risk of pancreatic cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol. 2010;172(1):81–93. doi: 10.1093/aje/kwq120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Purdue MP, Freedman DM, Gapstur SM, et al. Circulating 25-hydroxyvitamin D and risk of non-Hodgkin lymphoma: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol. 2010;172(1):58–59. doi: 10.1093/aje/kwq117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kutuzova GD, Deluca HF. 1,25-Dihydroxyvitamin D3 regulates genes responsible for detoxification in intestine. Toxicol Appl Pharmacol. 2007;218(1):37–44. doi: 10.1016/j.taap.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Xu H, McCann M, Zhang Z, et al. Vitamin D receptor modulates the neoplastic phenotype through antagonistic growth regulatory signals. Mol Carcinog. 2009;48(8):758–772. doi: 10.1002/mc.20520. [DOI] [PubMed] [Google Scholar]