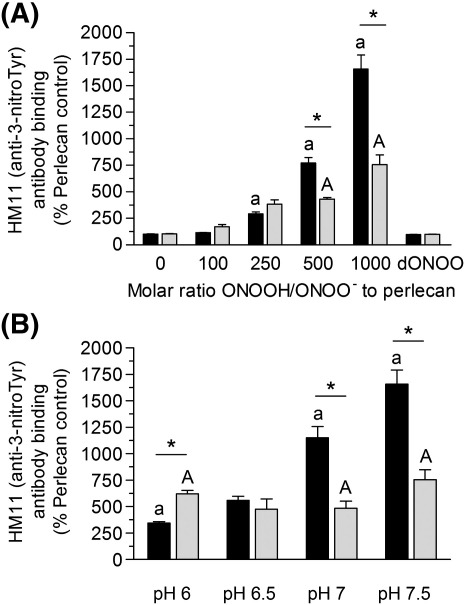
Fig. 3.
Formation of the tyrosine oxidation product 3-nitroTyr on perlecan exposed to peroxynitrite: (A) effect of oxidant concentration, and (B) effect of reaction pH. Surface absorbed perlecan (10 nM) was treated in the absence (black bars) and presence (light grey bars) of NaHCO3 (25 mM) for 20 min at 22 °C with (A) 0.1 M phosphate buffer, pH 7.5 containing 1 – 10 μM peroxynitrite (100 – 1000 molar excess of oxidant over protein) or dONOO (10 μM) or (B) 0.1 M phosphate buffer, pH 6 – 7.5 containing 10 μM peroxynitrite. The perlecan was then probed by ELISA using a mAb against 3-nitroTyr (HM11). Data are expressed as a % of the control ELISA signal obtained with untreated perlecan and are means ± SEM of values obtained from triplicate wells with n ≥ 3. The absolute absorbance (at 405 nm) for the controls with no perlecan were ∼ 0.055, and for native perlecan ∼ 0.09; those for the peroxynitrite-treated perlecan were up to 1.6 absorbance units. These data indicate that the level of 3-nitroTyr on the native perlecan was very low. Data were analyzed by 2-way ANOVA, with statistical significance assumed at p < 0.05; a/A: different to native perlecan in the absence or presence of NaHCO3 respectively, * effect of NaHCO3.