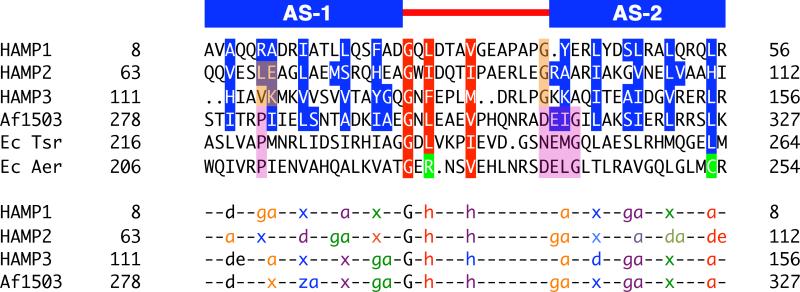
Figure 5. Residue Conservation and Packing Modes of HAMP Domains.
Sequence alignment of representative HAMP domains: Aer2, Af1503, EcTsr, and EcAer, showing conservation of the buried core (blue) and connector motif (red) residues (top panel). The bottom panel denotes the heptad position of core residues (a, d, e, g, z = undefined, x = directed at supercoil axis), as defined by TWISTER, in a variety of packing modes: a-d, x-da (x-ga or x-de), and x-x. Sets of interacting residues are color-coded to highlight the sidechains of HAMP1, HAMP3, and Af1503 (in-register) and HAMP2 (offset) (bottom panel). “G” and “h” correspond to conserved residues of the connector motif and are also color coded with the layer of interacting residues. Position of the conserved proline residue (purple) in canonical HAMP domains corresponds to residues that occupy different heptad positions (peach) in alternative HAMP conformations. Aer2 HAMPs conserve Gly residues (peach) at the beginning of AS2, which allows close association of domains in a poly-HAMP chain, compared to the DExG motif of canonical HAMPs (purple).