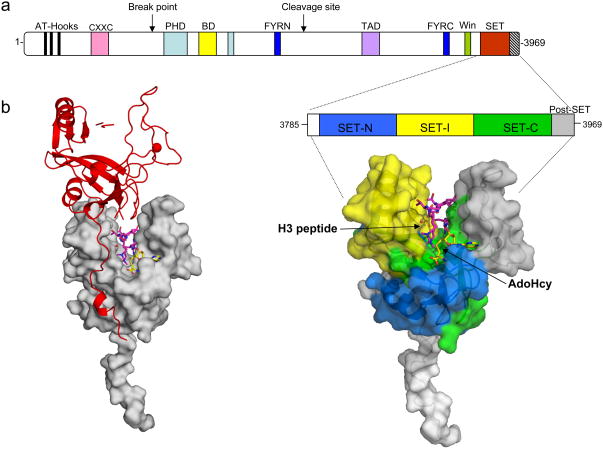
Figure 3.
X-ray crystal structure of the c-terminal MLL1 SET domain bound to s-adenosyl homocysteine (yellow) and histone H3 peptide (purple) (PDB code: 2W5Z). (a) At the top is a schematic representation of the full-length MLL1 protein and blown up is the construct used for crystallization of the MLL1 SET domain (residues 3785–3969). The SET-N, SET-I, and SET-C sub-domains are colored in blue, yellow and green, respectively. The post-SET domain is colored in grey, and the N-flanking region is colored white. The positions of histone H3 and AdoHcy are indicated. b) Crystal packing constrains the MLL1 SET domain into an open conformation. Surface representation of the MLL1 SET domain (grey) shown with a symmetry related molecule in red. The N-terminus of the symmetry related molecule interacts extensively with the SET-I region-constraining the MLL1 SET domain in an open conformation.