Abstract
Amphetamines induce stereotypy, which correlates with patch-enhanced c-Fos expression the patch compartment of caudate putamen (CPu). Methamphetamine (METH) treatment also induces patch-enhanced expression of prodynorphin (PD), arc and zif/268 in the CPu. Whether patch-enhanced activation of any of these genes correlates with METH-induced stereotypy is unknown, and the factors that contribute to this pattern of expression are poorly understood. Activation of mu opioid receptors, which are expressed by the neurons of the patch compartment, may underlie METH-induced patch-enhanced gene expression and stereotypy. The current study examined whether striatal mu opioid receptor blockade altered METH-induced stereotypy and patch-enhanced gene expression, and if there was a correlation between the two responses. Animals were intrastriatally infused with the mu antagonist CTAP (10 μg/μl), treated with METH (7.5 mg/kg, s.c.), placed in activity chambers for 3h, and then sacrificed. CTAP pretreatment attenuated METH-induced increases in PD, arc and zif/268 mRNA expression and significantly reduced METH-induced stereotypy. Patch-enhanced PD and arc mRNA expression in the dorsolateral CPu correlated negatively with METH-induced stereotypy. These data indicate that mu opioid receptor activation contributes to METH-induced gene expression in the CPu and stereotypy, and that patch-enhanced PD and arc expression may be a homeostatic response to METH treatment.
Keywords: psychostimulant, opioid, immediate early gene, caudate putamen, behavior
1. Introduction
Acute, as well as repeated treatment with psychostimulants, particularly the amphetamines (such as methamphetamine; METH) can induce stereotypy in humans and experimental animals. Stereotypy is defined as the development of abnormally repetitive motor actions that coincides with an inability to initiate normal adaptive responses (Canales and Graybiel, 2000; Graybiel and Canales, 2000; Graybiel et al., 2000; Graybiel and Rauch, 2000). In humans, these repetitive behaviors may be accompanied by repetitive patterns of attention, emotion and planning, similar to what is seen in those who suffer from Tourette syndrome or obsessive-compulsive disorder (Canales and Graybiel, 2000; Graybiel and Canales, 2000; Graybiel et al., 2000; Graybiel and Rauch, 2000). The relative activity of the patch (striosome) and matrix compartments of the rostral CPu may be related to the expression of psychostimulant-induced stereotypy. Enhanced expression of the immediate early gene (IEG) c-Fos in the patch compartment, relative to the surrounding matrix compartment in the dorsolateral aspects of rostral CPu has been shown to significantly correlate with the degree of stereotypy induced by amphetamine (AMPH; (Canales and Graybiel, 2000). The patch compartment possesses circuitry that tends to be limbic in nature, whereas the matrix compartment possesses circuitry that is thought to be predominantly non-limbic (Bolam et al., 1988; Gerfen, 1984, 1989; Ragsdale and Graybiel, 1988). Thus, it is thought that the enhanced activity of the limbic-associated circuits in the patch compartment relative to the non-limbic circuits of the matrix may underlie the expression of inflexible, internally-driven behaviors, such as stereotypy (Graybiel and Canales, 2000; Graybiel et al., 2000).
Treatment with high doses of AMPH or METH initially induces a patch-enhanced expression of the IEGs arc and zif/268 that becomes more diffuse over time, while dynorphin expression remains “patchy” for several hours after treatment (Adams et al., 2003; Fosnaugh et al., 1995; Gonzalez-Nicolini and McGinty, 2002; Horner and Keefe, 2006; Moratalla et al., 1992; Tan et al., 2000; Wang and McGinty, 1995; Wang et al., 1995). zif/268 codes for transcription factors that act on downstream target genes, whereas arc mRNA is trafficked to activated synapses (Cole et al., 1995; Lyford et al., 1995; Mildbrandt, 1987; Steward et al., 1998; Steward and Worley, 2001). Dynorphin content in the rostral CPu is increased during spontaneous stereotypic behavior (Presti and Lewis, 2005) and AMPH-induced IEG expression during stereotyped behavior occurs in dynorphin-positive neurons (Moratalla et al., 1992). Thus, activation of arc and zif/268 may be a first step in a chain of transcriptional events that influences long-term plasticity in neurons and along with dynorphin, could ultimately determine the acute behavioral response to treatment with amphetamines. However, it is not clear whether a relationship exists between patch-enhanced gene expression and METH-induced stereotypic behavior. Furthermore, the mechanisms that underlie METH-induced enhancement of gene expression in the patch compartment and stereotypy are poorly understood.
Activation of mu opioid receptors in the CPu may be necessary for the changes in gene expression in the patch compartment induced by amphetamines, as mu opioid receptors are expressed by the neurons of the patch compartment (Herkenham and Pert, 1981; Pert et al., 1976; Tempel and Zukin, 1987). Systemic treatment with the mu opioid receptor antagonist cloccinamox attenuated METH-induced increases in preprodynorphin mRNA expression in the rostral aspects of the patch compartment and intrastriatal infusion of a mu opioid receptor antagonist reduced AMPH-induced increases in neuropeptide expression in the CPu as a whole (Gonzalez-Nicolini et al., 2003; Horner and Keefe, 2006). While mu opioid receptor activation is typically associated with inhibition of neuronal activity, recent data indicates that mu opioid receptor activation can also lead to an enhancement of neuronal activity (Brami-Cherrier et al., 2005; Chavkin, 1988; Shoda et al., 2001). For example, mu opioid receptor activation can lead to an increase in mitogen activated protein kinase (MAPK) activity, which has been shown to contribute to AMPH-induced neuropeptide and mu agonist-induced IEG expression in the CPu (Shi and McGinty, 2006; Shoda et al., 2001; Ziółkowska et al., 2005). Thus, it is possible that mu opioid receptor activation has the ability drive gene expression within the CPu. However, it is not known whether activation of striatal mu opioid receptors contributes to METH-induced increases in arc and zif/268 expression in the patch compartment.
Activation of mu opioid receptors in the CPu may also contribute to the stereotypic behavior induced by amphetamines. Woo and co-workers (Woo et al., 1985) found that a low dose of AMPH that did not induce stereotypy when given alone, was able to induce marked stereotypic behavior when a mu opioid receptor agonist was infused directly into the rostral CPu prior to AMPH treatment. However, it is currently unknown whether activation of mu opioid receptors in the CPu contributes to METH-induced stereotypy.
The purpose of the present study was to test the hypothesis that striatal mu opioid receptor activation contributes to METH-induced stereotypy and increases in prodynorphin (PD) and IEG mRNA expression in the patch compartment by infusing the mu-specific antagonist CTAP (H-D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2; (Kramer et al., 1988) directly into the CPu prior to METH treatment. We then examined METH-induced stereotypy and increases in PD, arc and zif/268 mRNA in the patch and matrix compartments of the CPu using in situ hybridization and immunohistochemistry. Finally, we determined whether the degree of patch-enhanced expression of PD, arc or zif/268 correlated significantly with the severity of METH-induced stereotypy and whether mu opioid receptor activation contributed to this potential relationship.
2. Materials and methods
2.1. Animals and Surgery
Male Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN, USA), weighing 250-350g were used in all experiments. Rats were housed in groups of four in plastic cages in a temperature-controlled room. Rats were on a 14:10 h light/dark cycle and had free access to food and water. All animal care and experimental manipulations were approved by the Institutional Animal Care and Use Committee of Mercer University School of Medicine and were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The minimum possible number of animals (based on power analyses) was used for our experiments and steps were taken to minimize any suffering that might occur during our procedures.
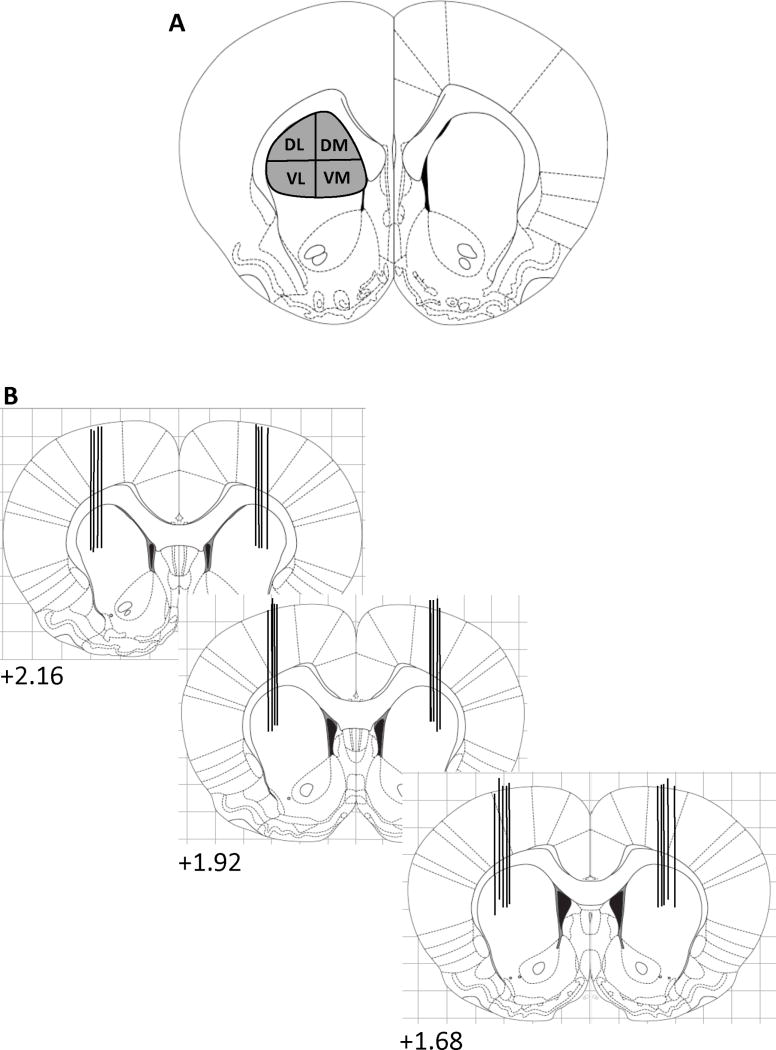
Five to seven days prior to the experiment, rats were anesthetized with ketamine (90 mg/kg, i.p.) and xylazine (9 mg/kg, i.p.) and stainless steel 26-gauge guide cannulae 3.5 mm in length (Plastics One, Roanoke, VA, USA) were implanted bilaterally, based on the coordinates of Paxinos and Watson (Paxinos and Watson, 2005) in the rostral striatum (+2.16 to +1.68 mm from bregma, approximately ±2.6 mm lateral to midline and 3.5 mm below the surface of the skull (Fig. 1). The guide cannulae were kept patent with 31-gauge obturators that were the same length as the guides. In order to minimize acute tissue damage and spurious IEG expression during the experiment, the day before the experiment the original dummy cannulae were removed and replaced with dummy cannulae that extended 2.0 mm beyond the guide cannulae (Adams et al., 2000; Keefe and Gerfen, 1995). The day of the experiment, the dummy cannulae were removed and 31-gauge stainless steel injection cannulae that extended 1.5 mm beyond the guide were inserted into the guide cannulae. A 1-μl volume of buffered aCSF (144 mM NaCl; 2.68 mM KCl; 1.6 mM CaCl2; 2.6 mM MgCl2; 0.4 mM KH2PO4, pH, 7.2) or the mu opioid receptor antagonist CTAP (10 μg/μl, a dose determined by pilot studies in our laboratory; (Kramer et al., 1988) was administered bilaterally at a rate of 0.1 μl/min to the freely moving animal. After each infusion, the injection cannulae were left in place for 5 min in order to minimize fluid back flow through the cannulae. Only animals whose cannulae were in the rostral striatum were included in subsequent analyses.
Figure 1.
Schematic diagram of the four sub-regions in rostral (AP+ 1.68) CPu used for autoradiogram analysis of mRNA expression in the patch and matrix compartments (A) and the approximate locations of the microinjection needle tips (B). The numbers indicate the millimeters anterior to bregma (Paxinos and Watson, 2005). DL, dorsolateral, DM, dorsomedial, VL, ventrolateral, VM, ventromedial (Adapted from Adams et al., 2001 and Gonzalez-Nicolini et al., 2003).
2.2. Experimental Design and Behavior
Twenty-four hours prior to the experiment, animals were habituated to plexiglass activity chambers (46 × 46 × 12 cm, on top of a 4 × 4 grid; (Frankel et al., 2007) by placing them in the chambers for 60 min, giving them sham injections and returning them to the chambers for 3h. The next day, animals were placed in the chambers for 60 min, after which time they were bilaterally infused with either aCSF or 10 μg/μl CTAP. The animals were then injected with METH (7.5 mg/kg, s.c.) or saline and returned to the activity chambers for 3h, during which time the behavior was digitally recorded for post-hoc analyses. During the post-hoc analyses, each animal was observed for 1 min every 5 min for the entire 3h period after the injection of METH or saline by an observer blind to the experimental conditions. Stereotypy was rated on a scale of 1-10, with 10 representing the highest degree of the response (Canales and Graybiel, 2000; Creese and Iverson, 1973). Stereotypy scores were generated by averaging the scores from four behavioral dimensions: repetitiveness/flexibility (the number of alternative motor responses emitted), frequency (the number of responses per unit time), duration (the percentage of time spent performing the most dominant response(s)) and the spatial distribution of the motor response (Canales and Graybiel, 2000).
2.3. In Situ Hybridization Histochemistry
Three hours after treatment with METH or saline, rats were sacrificed by exposure to CO2 for 1 min followed by decapitation. The brains were rapidly harvested and quick-frozen in isopentane on dry ice. Brains were stored at -80°C until they were cut into 12-μm sections on a cryostat (Minotome Plus, Triangle Biomedical Sciences, Durham, N.C., USA). Sections were thaw-mounted onto Superfrost slides (VWR, Westchester, PA, USA) and stored at -20°C. Slides from all animals were then post-fixed in 4% paraformaldehyde/0.9% NaCl, acetylated in fresh 0.25% acetic anhydride in 0.1 M triethanolamine/0.9% NaCl (pH 8.0), dehydrated in alcohol, delipidated in chloroform and gradually re-hydrated in a descending series of alcohol concentrations. Slides were air-dried and stored at -20°C.
For the detection of PD mRNA, an oligonucleotide probe (GeneDetect, Bradenton, FL, USA) that was complementary to bases 762-809 of the PD mRNA (Civelli et al., 1985) was end-labeled with [35S]-dATP (Perkin Elmer NEN, Wellesley, MA, USA), as previously described (Adams et al., 2003; Horner et al., 2005; Horner and Keefe, 2006; Horner et al., 2009). Briefly, the probe was diluted in hybridization buffer (0.6 M sodium chloride, 80 mM Tris, 4 mM EDTA, 0.1% w/v sodium pyrophosphate, 10% w/v dextran, 0.2% w/v lauryl sulfate, 0.5 mg/ml heparin, 50% formamide) and 90 μl of the probe in hybridization buffer was applied to each slide and covered with glass coverslips. Slides were hybridized overnight in humid chambers at 37°C. The slides were then washed four times in 1× saline-sodium citrate (SSC; 0.15 M NaCl, 0.015 M sodium citrate, pH 7.2) at room temperature and then three times in 2× SSC with 50% (v/v) formamide at 42°C. Slides were washed twice in 1× SSC at room temperature, rinsed briefly in deionized H2O and air-dried.
Full-length ribonucleotide probes were used for the detection of zif/268 (Mildbrandt, 1987) and arc mRNA (Lyford et al., 1995), as previously described (Adams et al., 2001; Horner and Keefe, 2006). The probe was synthesized from the cDNA using [35S]-UTP (Perkin Elmer, NEN, Wellesley, MA, USA) and the appropriate RNA polymerase. The probe was mixed with nuclease-free water and (final concentrations) salmon sperm DNA (200 μg/μl), yeast total RNA (250 μg/μl) and tRNA (250 μg/μl). The solutions were then denatured at 65°C for 5 min and cooled on ice for 1 min. The following compounds were added to the solution (final concentrations): dithiothreitol (100 mM), sodium dodecyl sulfate (0.2% w/v), sodium thiosulfate (0.11% w/v) and hybridization buffer (23.8 mM Tris, pH 7.4; 1.2 mM EDTA, pH 8.0; 357 mM sodium chloride; dextran, 11.9% w/v; 1.2 × Denhardt's solution; 59.5% w/v formamide) to the appropriate dilution. Hybridization buffer containing probe (2 × 106 cpm/100 μl buffer) was added to the slides as described above, and incubated overnight in humid chambers at 55°C. The slides were then washed four times in 1 × SSC at room temperature, then in buffer (0.5 M sodium chloride, 5 mM Tris and 250 μM EDTA) containing RNAse A (5 μg/ml; Roche Biomedical Laboratories, Burlington, NC, USA). RNAse A treatment was followed by four washes in 0.2× SSC at 60°C. The slides were then dipped in deionized H2O and air-dried.
All labeled slides were apposed to X-ray film (Kodak Biomax MR film, Kodak Company, Rochester, NY, USA) for approximately 30 days.
2.4. Mu Opioid Receptor Immunohistochemistry
Immunohistochemistry for mu opioid receptors was performed as previously described (Adams et al., 2003; Horner et al., 2005; Horner and Keefe, 2006; Horner et al., 2009), using serial, 12-μm sections through the CPu that were adjacent to those used for in situ hybridization. Sections were post-fixed in 4% paraformaldehyde/0.9% NaCl and then rinsed three times in 0.1 M phosphate-buffered saline (PBS). Slides were then blocked with 10% bovine serum albumin (BSA)/0.3% Triton X-100 (TX)/0.1 M PBS for 2 h followed by overnight incubation at 4°C with a polyclonal antibody for the mu opioid receptor (Immunostar, Hudson, WI, USA), diluted in 1:1000 in 0.3% TX/0.1M PBS/5% BSA. The slides were then washed several times in PBS and incubated for 2 h at room temperature in biotinylated goat anti-rabbit IgG antiserum (Vector Laboratories, Burlingame, CA, USA) diluted 1:200 in 0.1M PBS/5% BSA. Slides were then washed three times in PBS, incubated 1 h in ABC solution (Elite ABC Kit, Vector Laboratories) and washed three more times in PBS. Bound antibody was detected using a 3′,3-diaminobenzidine/Ni+ solution (Vector Laboratories). Slides were washed with deionized H2O, dehydrated in a series of alcohols and coverslipped out of xylene.
2.5. Film Analysis
Film autoradiograms were analyzed using the image analysis program ImageJ (National Institutes of Health; http://rsb.info.nih.gov/ij), as previously described (Adams et al., 2003; Horner et al., 2005; Horner and Keefe, 2006; Horner et al., 2009). Basic densitometric analysis yielded average density (gray) values over regions of interest. Before the measurement of sections, the linearity of the video camera to increasing signal intensity was determined using the average gray values of signals of known optical density from a photographic step tablet (Eastman Kodak Company, Rochester, NY, USA). The intensity of the light was adjusted such that the values measured from the film autoradiograms of brain sections fell within the linear portion of the system's response.
The images of sections from all groups within an experiment that were processed and hybridized in parallel were captured and measured under constant lighting and camera conditions. Images from 5 to 8 animals in each treatment group were analyzed for each mRNA and one section per animal was analyzed for each region of interest examined. Measurements were made according to the coordinates of Paxinos and Watson (Paxinos and Watson, 2005) in the left hemisphere of the rostral CPu (+2.16 to +1.68 mm anterior to bregma). The average gray value of the white matter overlying the structure being measured was subtracted from the average gray value of the region of interest to correct for background labeling.
In order to distinguish the patch and matrix compartments of the CPu, sections adjacent to those used for in situ hybridization for PD, arc or zif/268 mRNA were processed for mu opioid receptor immunohistochemistry. Measurements were made in the patch and matrix of the rostral CPu, as previously described (Adams et al., 2003; Horner et al., 2005; Horner and Keefe, 2006; Horner et al., 2009), and encompassed four sub-regions (Adams et al., 2001): dorsolateral (DL), dorsomedial (DM), ventrolateral (VL) and ventromedial (VM) CPu (Fig. 1A). Immunohistochemically labeled sections were captured at the same magnification as the in situ hybridization-labeled sections. Patches of mu opioid receptor immunoreactivity were outlined using the ImageJ software and superimposed over corresponding areas on the in situ hybridization-labeled CPu sections and analyzed as described above. Areas where mu opioid receptor immunoreactivity was absent were analyzed as a measure of mRNA expression in the matrix compartment of CPu. A ratio of patch-to-matrix mRNA expression (Canales and Graybiel, 2000; Horner et al., 2009) was calculated for each mRNA in each of the four sub-regions of the rostral CPu and was accomplished by dividing the average gray value of the patch by the average gray value of the matrix, for each animal in the study.
2.6. Statistical Analysis
The effects of mu opioid receptor blockade on PD, arc and zif/268 mRNA expression in the rostral CPu induced by METH was analyzed using a two-way (pre-treatment × acute treatment) analysis of variance. A separate analysis was performed for each compartment in each of the four sub-regions of the rostral CPu. Behavioral rating data was represented as the area under the curve (AUC) and was also analyzed using a two-way (pre-treatment × acute treatment) analysis of variance. Post-hoc analysis of significant effects was accomplished using individual Bonferroni (Dunn) t-tests. The alpha level for all analyses was set at 0.05. In the case of a significant overall main effect of treatment, the alpha level was not corrected, as only one comparison was made in the post-hoc analysis (saline vs. METH). For the post-hoc analysis of significant interaction terms, four comparisons were made (vehicle/saline vs. vehicle/METH; vehicle/saline vs. CTAP/saline; vehicle/METH vs. CTAP/METH; CTAP/saline vs. CTAP/METH), thus requiring a P-value=0.0125 (0.05/4) for statistical significance. Correlations between the ratio of patch-to-matrix gene expression in each of the four sub-regions of the rostral CPu for each mRNA and the cumulative stereotypy score for the entire 3h observation period, were calculated according to the Spearman method, with statistical significance set at P<0.05.
2.7. Drugs
(±)METH hydrochloride was a generous gift from the National Institute on Drug Abuse (Bethesda, MD, USA). Ketamine hydrochloride and xylazine hydrochloride were obtained from Sigma Aldrich (St. Louis, MO, USA). METH, ketamine and xylazine doses were calculated as the free base and dissolved in normal saline. All drugs were given in a volume of 1 ml/kg. CTAP was obtained from Sigma Aldrich (St. Louis, MO, USA) and dissolved in aCSF.
3. Results
3.1. Effects of striatal mu opioid receptor blockade on METH-induced PD mRNA expression in the CPu
In CPu of vehicle-pretreated animals, METH-induced increases in PD mRNA were somewhat “patchy” in appearance, while in CTAP-pretreated animals, the effects of METH on PD mRNA expression were reduced (Fig. 2A and B). Two-way analysis of variance of the effects of striatal mu opioid receptor blockade on METH-induced PD mRNA expression revealed significant effects of pretreatment and treatment as well as significant pretreatment × treatment interactions in both the patch and matrix compartments of DL, DM and VM CPu, but not the VL CPu (Fig. 2C-F). Post-hoc analysis revealed that METH significantly increased PD mRNA expression in the patch compartment of DL (t=4.66, P=0.0007), DM (t=3.83, P=0.003) and VM (t=4.60, P=0.0008) CPu, as well as the matrix compartment of DL (t=4.29, P=0.001), DM (t=4.17, P=0.002) and VM (t=3.97, P=0.002) CPu. METH did not significantly increase PD mRNA expression in CTAP-pretreated animals in the patch compartment of DL (t=1.74, P=0.12), DM (t=0.33, P=0.75) and VM (t=0.29, P=0.78) CPu, or in the matrix compartment of DL (t=1.56, P=0.16), DM (t=0.65, P=0.54) and VM (t=0.42, P=0.67) CPu. Unexpectedly, in the VL CPu, CTAP pretreatment resulted in a decrease in PD mRNA expression that was below that observed for vehicle-pretreated control animals. Two-way analysis of variance revealed a significant effect of pretreatment and a significant pretreatment × treatment interaction, but not a significant main effect of treatment in this region.
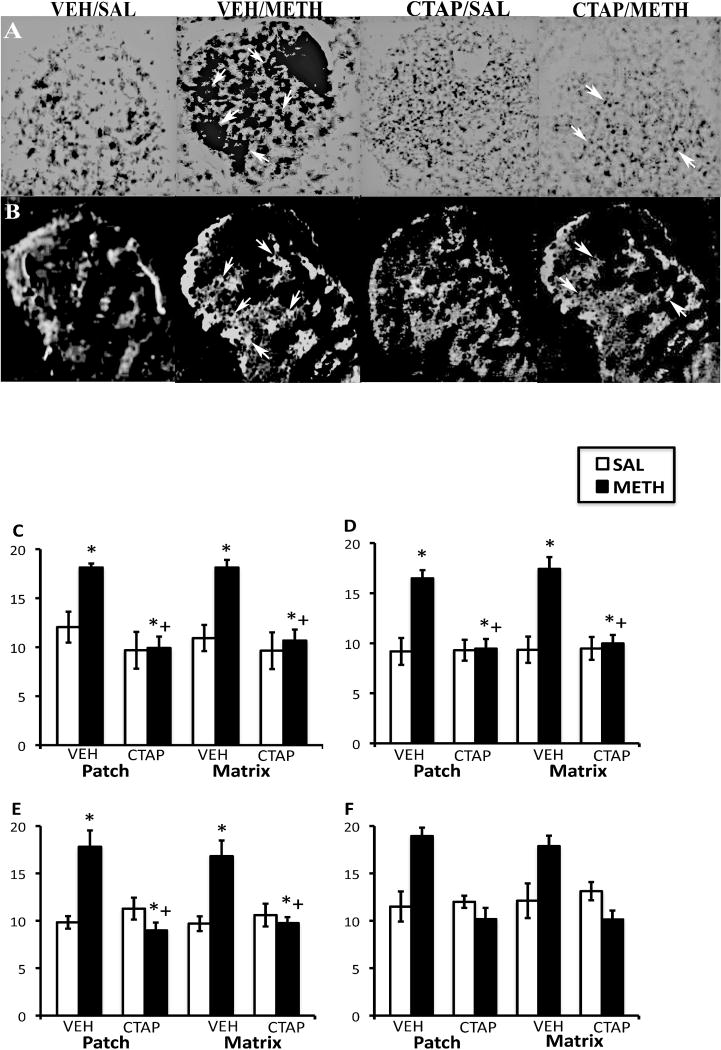
Figure 2.
Effects of CTAP pretreatment on METH-induced PD mRNA expression in the rostral CPu. In situ hybridization film autoradiograms showing PD (A) and mu opioid receptor immunohistochemical staining in adjacent sections (B) and quantitative analysis of PD mRNA expression in the patch and matrix compartments of DL (C), DM (D), VM (E) and VL (F) CPu, from rats intrastriatally infused with vehicle or CTAP (10 μg/μl), 15 min prior to METH or saline treatment. Arrows indicate patches of mu opioid receptor labeling and corresponding patches of intense PD mRNA expression. Note the decrease in PD expression in METH-treated animals pretreated with CTAP. Quantitative values are average gray values (arbitrary units; ±S.E.M., n=5–8 animals/group) obtained from densitometric analysis of film autoradiograms. *Significantly different from saline-pretreated control group, P<0.05; +Significantly different from respective saline-pretreated group, P<0.05. There was a not significant main effect of treatment in the VL CPu, but a significant effect of pretreatment and a significant pretreatment-treatment interaction.
3.2. Effects of striatal mu opioid receptor blockade on METH-induced arc mRNA expression in the CPu
Overall, METH-induced increases in arc mRNA expression in the CPu appeared to be homogeneous in vehicle-pretreated animals and CTAP pretreatment reduced METH-induced arc mRNA expression (Fig. 3A and 3B). Two-way analysis of variance of the effects of striatal mu opioid receptor blockade on METH-induced arc mRNA expression revealed significant effects of pretreatment and treatment as well as significant pretreatment × treatment interactions in both the patch and matrix compartments of DL, DM, VM and VL CPu (Fig. 3C-F). Post-hoc analysis revealed that METH significantly increased arc mRNA expression in the patch compartment of DL (t=8.52, P<0.0001), DM (t=5.60, P=0.0003), VM (t=5.47, P=0.0004) and VL (t=6.91, P<0.0001) CPu, as well as the matrix compartment of DL (t=9.54, P<0.0001), DM (t=5.60, P=0.0003), VM (t=6.74, P<0.0001) and VL (t=7.43, P<0.0001) CPu. METH treatment did not significantly increase arc mRNA expression in CTAP-pretreated animals in the patch compartment of DL (t=0.46, P=0.66), DM (t=0.62, P=0.56), VM (t=2.16, P=0.06) and VL (t=0.58, P=0.57) CPu, or in the matrix compartment of DL (t=1.01, P=0.330), DM (t=0.83, P=0.43) VM (t=2.9, P=0.02) and VL (t=0.36, P=0.73) CPu.
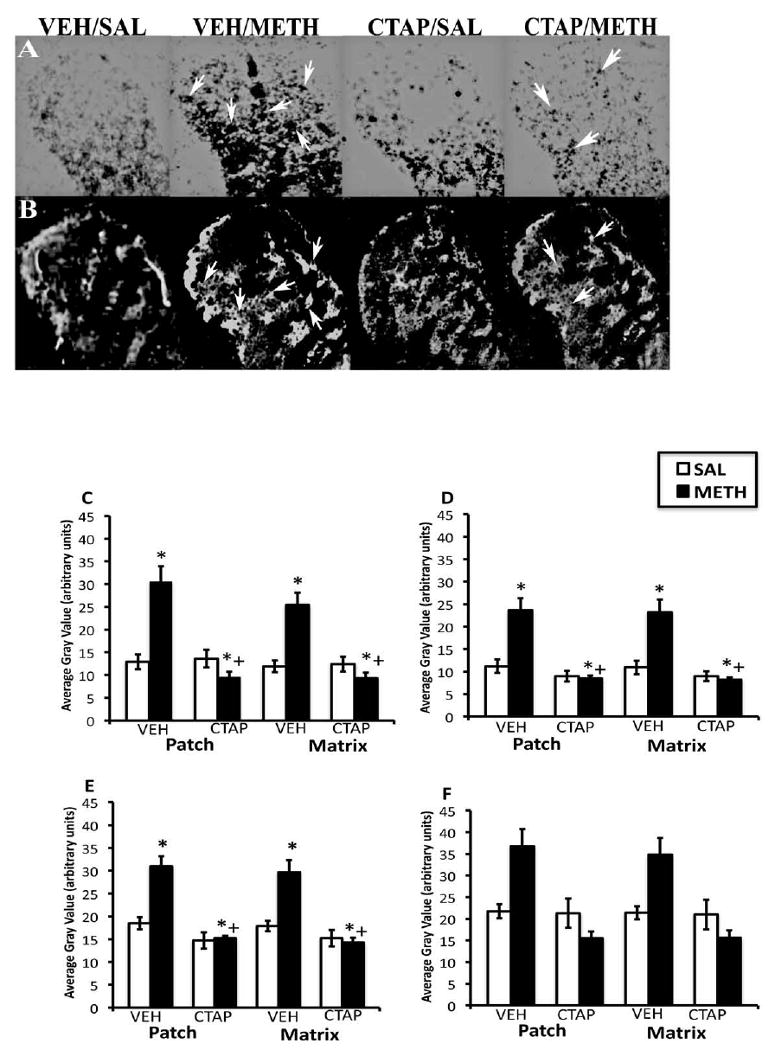
Figure 3.
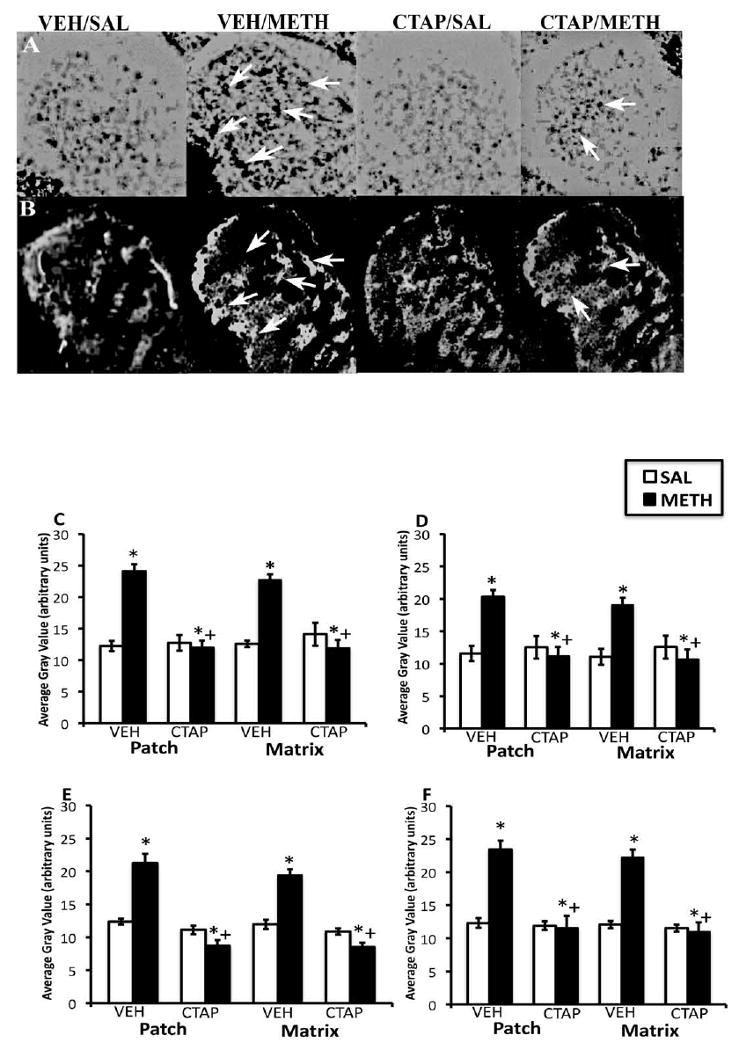
Effects of CTAP pretreatment on METH-induced arc mRNA expression in the rostral CPu. In situ hybridization film autoradiograms showing arc (A) and mu opioid receptor immunohistochemical staining in adjacent sections (B) and quantitative analysis of arc mRNA expression in the patch and matrix compartments of DL (C), DM (D), VM (E) and VL (F) CPu, from rats intrastriatally infused with vehicle or CTAP (10 μg/μl), 15 min prior to METH or saline treatment. Arrows indicate patches of mu opioid receptor labeling and corresponding patches of intense arc mRNA expression. Note the decrease in arc expression in METH-treated animals pretreated with CTAP. Quantitative values are average gray values (arbitrary units; ±S.E.M., n=5–8 animals/group) obtained from densitometric analysis of film autoradiograms. *Significantly different from saline-pretreated control group, P<0.05; +Significantly different from respective saline-pretreated group, P<0.05.
3.3. Effects of striatal mu opioid receptor blockade on METH-induced zif/268 mRNA expression in the CPu
In CPu of vehicle-pretreated animals, METH-induced increases in zif/268 mRNA were diffuse in appearance, while in CTAP-pretreated animals, the effects of METH on zif/268 mRNA expression were reduced (Fig. 4A and 4B). Two-way analysis of variance of the effects of striatal mu opioid receptor blockade on METH-induced zif/268 mRNA expression revealed significant effects of pretreatment and treatment as well as significant pretreatment × treatment interactions in both the patch and matrix compartments of DL, DM and VM CPu (Fig. 4C-F). Post-hoc analysis revealed that METH significantly increased zif/268 mRNA expression in the patch compartment of DL (t=4.012, P=0.002), DM (t=4.80, P=0.0006) and VM (t=4.05, P=0.002) CPu, as well as the matrix compartment of DL (t=4.83, P=0.0005), DM (t=4.58, P=0.0008) and VM (t=3.70, P=0.003) CPu. METH did not significantly increase zif/268 mRNA expression in CTAP-pretreated animals in the patch compartment of DL (t=0.113, P=0.91), DM (t=0.113, P=0.91) and VM (t=1.709, P=0.133) CPu, and in the matrix compartment of DL (t=0.50, P=0.63), DM (t=0.37, P=0.72) and VM (t=0.65, P=0.53) CPu. Similar to what was observed for PD mRNA expression, in the VL CPu, CTAP pretreatment resulted in a decrease in zif/268 mRNA expression that was below that observed for vehicle-pretreated control animals. Two-way analysis of variance revealed a significant effect of pretreatment and a significant pretreatment × treatment interaction, but not a significant main effect of treatment in this region.
Figure 4.
Effects of CTAP pretreatment on METH-induced zif/268 mRNA expression in the rostral CPu. In situ hybridization film autoradiograms showing zif/268 (A) and mu opioid receptor immunohistochemical staining in adjacent sections (B) and quantitative analysis of PD mRNA expression in the patch and matrix compartments of DL (C), DM (D), VM (E) and VL (F) CPu, from rats intrastriatally infused with vehicle or CTAP (10 μg/μl), 15 min prior to METH or saline treatment. Arrows indicate patches of mu opioid receptor labeling and corresponding patches of zif/268 mRNA expression. Note the decrease in zif/2678 expression in METH-treated animals pretreated with CTAP. Quantitative values are average gray values (arbitrary units; ±S.E.M., n=5–8 animals/group) obtained from densitometric analysis of film autoradiograms. *Significantly different from saline-pretreated control group, P<0.05; +Significantly different from respective saline-pretreated group, P<0.05. There was not a significant main effect of METH treatment in the VL CPu.
3.4. Effects of striatal mu opioid receptor blockade on METH-induced stereotypy
Acute METH treatment resulted in an initial increase in activity during the first 30-45 min after treatment, which was followed by a high degree of stereotypic behavior, an effect that was reduced by pretreatment with CTAP (Fig. 5A). Two-way analysis of variance of the stereotypy AUC values for the 3h observation period indicated that there was a significant main effect of CTAP pretreatment (F1,29=69, P<0.0001), METH treatment (F1,29=730, P<0.0001) and a pretreatment × treatment interaction (F1,29=80, P<0.0001). Post-hoc analysis revealed that METH induced significant stereotyped behavior in both vehicle- (t=21.37, P<0.0001) and CTAP-(t=17.39, P<0.0001) pretreated animals; however, the ability of METH to induce stereotypic behavior was reduced in CTAP-pretreated versus vehicle-pretreated animals (t=11.95, P<0.0001). Since a lack of mobility is characteristic of the stereotypic behavior induced by amphetamines (Creese and Iverson, 1973), we examined the spatial distribution scores from each group of animals (Fig. 5B). Two-way analysis of the AUC values for spatial distribution scores during the 3h observation period revealed a significant main effect of CTAP pretreatment (F1,29=31.5, P<0.0001), METH treatment (F1,29=233.0, P<0.0001) and a pretreatment × treatment interaction (F1,29=38.5, P<0.0001). Post-hoc analysis indicated that METH treatment resulted in significant immobility in both vehicle- (t=12.67, P<0.0001) and CTAP- (t=8.93, P<0.0001) pretreated animals, but the ability of METH to induce immobility was reduced in CTAP- versus vehicle-pretreated animals (t=8.37, P<0.0001). CTAP treatment alone did not significantly alter behavior.
Figure 5.
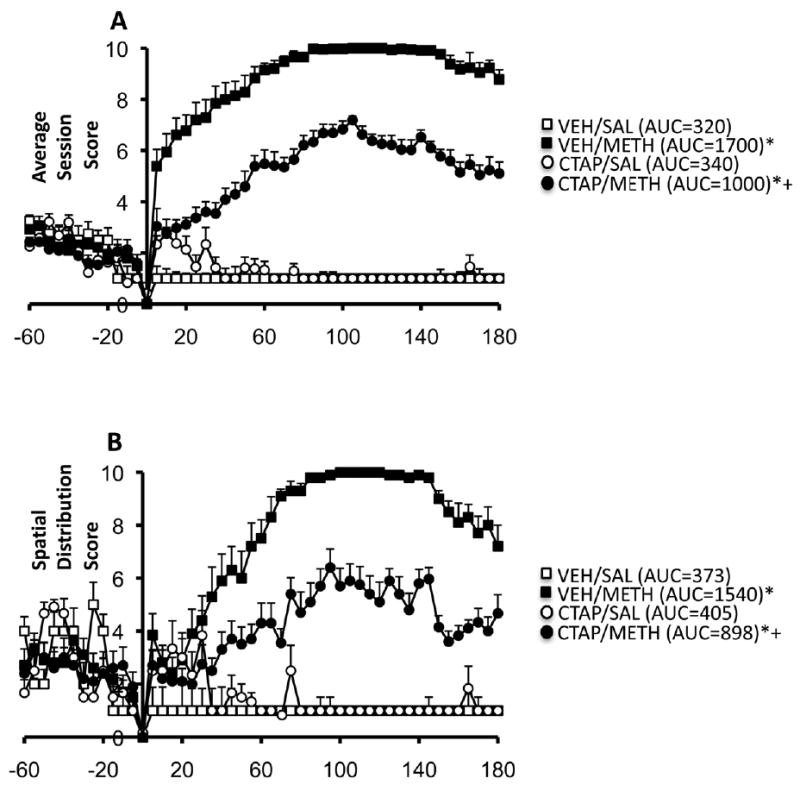
Effects of intrastriatal infusion of CTAP (10 μg/μl) and acute METH treatment (7.5 mg/kg) on stereotyped behavior (A) and spatial distribution scores (B). Values are expression as the mean ±SEM. AUC values are in parentheses. *Significantly different from respective control group, P<0.005; +Significantly different from vehicle-pretreated METH group, P<0.005. Acute METH treatment increased stereotypy and spatial distribution scores, both of which were reduced by pretreatment with CTAP.
3.6 Analysis of patch-enhanced gene expression and METH-induced stereotypic behavior
Two-way analysis of variance of the patch-to-matrix ratio of PD mRNA expression revealed that the pattern of PD mRNA expression the CPu was patch-enhanced, but only in the DL CPu (Fig 6A, inset). In the DL CPu, there was a significant main effect of CTAP pretreatment (F1,18=18, P=0.0005), METH treatment (F1,18=4.8, P=0.04) and a significant pretreatment × treatment interaction (F1,18=20, P=0.0003). Post-hoc analysis indicated that there was a significant difference in the ratio of patch-to-matrix PD mRNA expression in the DL CPu of vehicle-pretreated, METH-treated versus vehicle-pretreated, saline-treated animals (t=5.20, P=0.0004), an effect that was abolished by pretreatment with CTAP (t=1.28, P=0.236). Two-way analysis of variance also revealed the ratio of patch-to-matrix arc mRNA expression the CPu induced by METH treatment was patch-enhanced in the DL CPu (Fig 6B, inset), but not in other sub-regions of the CPu or for any other treatment groups. CTAP pretreatment did not significantly alter the patch-enhanced pattern of arc mRNA expression in the DL CPu, as there was a significant main effect of METH treatment (F1,18=4.9, P=0.04), but not a significant effect of CTAP pretreatment (F1,18=1.3, P=0.27) or a significant pretreatment × treatment interaction (F1,18=0.002, P=0.97). METH-induced zif/268 mRNA expression was not patch-enhanced in any sub-region of the CPu, as there was not a significant difference in the patch-to-matrix ratio of zif/268 mRNA expression between saline- and METH-treated groups (data not shown).
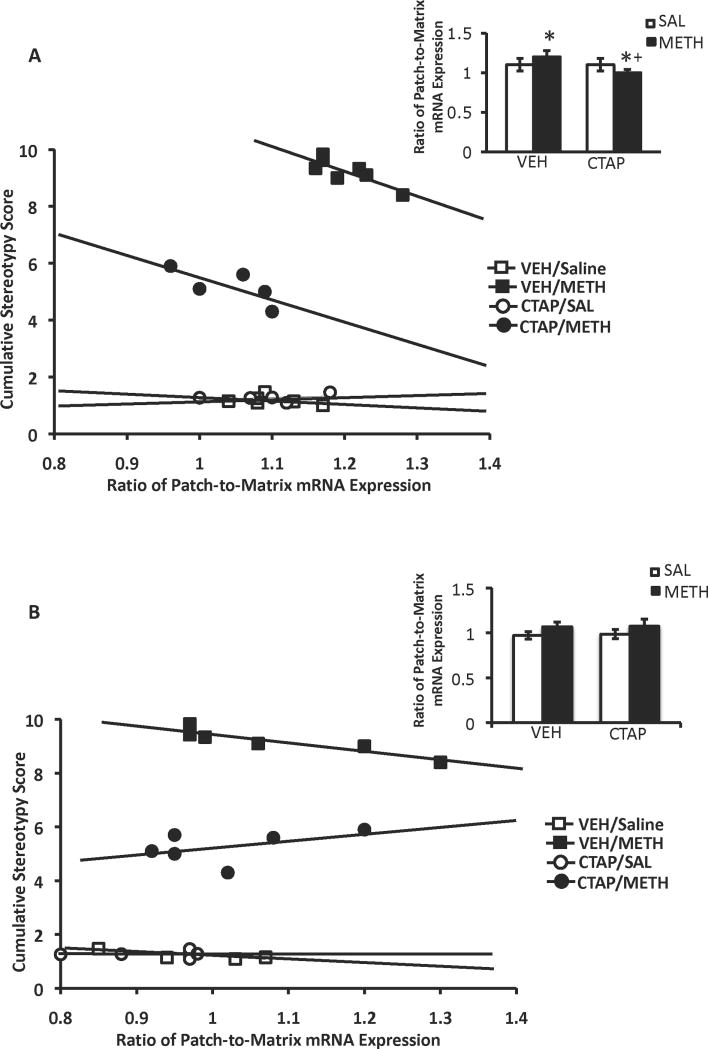
Figure 6.
Patch-enhanced gene expression in the DL CPu, and the correlation between cumulative stereotypy scores and the ratio of patch-to-matrix mRNA in the DL CPu. Acute METH treatment significantly increased the ratio of patch-to-matrix PD mRNA expression (A, inset) and arc mRNA expression (B, inset) only in the DL CPu an effect that was blocked by pretreatment with CTAP, for PD, but not arc mRNA expression. There was a significant negative correlation between the cumulative stereotypy scores and the ratio of patch-to-matrix expression of PD (A) and arc (B) mRNA expression, which was disrupted in both cases by mu striatal opioid receptor blockade. *Significantly different from saline-pretreated control group, P<0.05; +Significantly different from respective saline-pretreated group, P<0.05. There was an overall main effect of treatment for the patch-to-matrix ratio of arc mRNA expression.
In order to determine whether a relationship exists between the degree of gene expression in the patch versus matrix compartments and METH-induced stereotyped behavior, we determined if there was a correlation between the ratio of patch-to-matrix PD, arc and zif/268 mRNA expression in the four sub-regions of CPu and the cumulative stereotypy scores for the entire 3h session for each animal. In the DL sub-region of the CPu, there was a significant negative correlation between cumulative stereotypy scores and the ratio of patch-to-matrix PD mRNA expression in vehicle-pretreated/METH-treated animals (rs=-0.775; P=0.04), but not in vehicle-pretreated/saline-treated animals (rs=-0.406; P=0.42), CTAP-pretreated/saline-treated animals (rs=0.300, P=0.68) or CTAP-pretreated/METH-treated animals (rs=-0.73, P=0.162; Fig. 6A). There was no correlation between the cumulative stereotypy score and the ratio of patch-to-matrix PD mRNA expression in any other region of CPu, for any treatment group, except for in the DM CPu (data not shown). In this region, there was a significant positive correlation between the ratio of patch-to-matrix PD mRNA expression and cumulative stereotypy scores in CTAP-pretreated/saline-treated animals (rs=1.00, P=0.017; data not shown).
There was also a significant negative correlation in the DL CPu between cumulative stereotypy scores and the ratio of patch-to-matrix arc mRNA expression in vehicle-pretreated/METH-treated animals (rs=0.99, P=0.003), but not in vehicle-pretreated/saline-treated animals (rs=-0.500, P=0.45), CTAP-pretreated/saline-treated animals (rs=0.41, P=0.52) or CTAP-pretreated/METH-treated animals (rs=0.41, P=0.42; Fig. 6B). There was no significant correlation between cumulative stereotypy scores and the patch-to-matrix ratio of arc mRNA expression in any other sub-region of CPu for any treatment group (data not shown).
There was no significant correlation between cumulative stereotypy scores and the patch-to-matrix ratio of zif/268 mRNA expression in any sub-region of CPu for any treatment group (data not shown).
4. Discussion
The goal of this study was to determine if striatal mu opioid receptor activation contributes to METH-induced gene expression in the patch compartment and stereotypy. Striatal mu opioid receptor blockade inhibited METH-induced increases in PD, arc and zif/268 mRNA expression in both the patch and matrix compartments and significantly reduced METH-induced stereotypy. We also sought to determine if the patch-enhanced pattern of gene expression induced by METH correlated significantly with stereotypy and the effect of striatal mu opioid receptor blockade on this relationship. There was a significant negative correlation between patch-enhanced PD and arc mRNA expression in the DL CPu and METH-induced stereotypy and striatal mu opioid receptor blockade reduced this negative correlation. These data demonstrate a role for mu opioid receptor activation in METH-induced gene expression and stereotypy and are among the first to demonstrate a negative correlation between METH-induced patch-enhanced PD and arc mRNA expression in the DL CPu and stereotypic behavior.
METH-induced gene expression in the patch and matrix compartments
The dose of METH used in the current study increased PD, arc and zif/268 expression in both the patch and matrix compartments of all four sub-regions of rostral CPu examined. These data are in conflict with previous work where METH treatment significantly increased preprodynorphin mRNA expression in the patch, but not matrix compartment of rostral CPu (Adams et al., 2003; Horner et al., 2005; Horner and Keefe, 2006), but are in line with previous work showing that at this time point after METH or AMPH treatment, zif/268 and arc mRNA expression is homogeneous (Adams et al., 2001; Gonzalez-Nicolini and McGinty, 2002; Horner et al., 2005; Horner and Keefe, 2006; Wang and McGinty, 1995; Wang et al., 1995). It is also important to note that the dose of METH used in the current study (7.5 mg/kg) is half that used in our previous studies (Horner et al., 2005; Horner and Keefe, 2006). Treatment with a low dose of METH (2.0 mg/kg) does not significantly alter preprodynorphin gene expression in either compartment, whereas a moderate dose (7.5 mg/kg) increases gene expression in both compartments, and high doses of METH (15 mg/kg) or repeated treatment with AMPH appears to down-regulate gene expression in the matrix (Adams et al., 2003; Canales and Graybiel, 2000; Graybiel et al., 2000). Thus, the dose of METH used in our study was high enough to induce gene expression in the CPu overall, but not high enough to yield a suppression of gene expression in the matrix compartment as a whole.
Correlation between METH-induced patch-enhanced gene expression and stereotypy
Despite increasing gene expression in both compartments, the dose of METH used in our study was able to induce a patch-enhanced pattern of PD and arc mRNA expression in the DL CPu. This finding is of particular interest, since the DL CPu has been implicated in the expression of inflexible behaviors (Canales and Graybiel, 2000; Yin and Knowlton, 2006) and enhanced activation of c-Fos in the patch vs. matrix compartment of this region is thought to underlie AMPH-induced stereotypy (Canales and Graybiel, 2000). However, our data show that patch-enhanced PD and arc mRNA expression in the DL CPu correlated negatively with cumulative METH-induced stereotypy, which raises the possibility that patch-enhanced PD and arc mRNA expression in the DL CPu is not related to the induction METH-induced stereotypic behavior, but is a response to overstimulation of striatal neurons by METH. For example, METH-induced dynorphin expression in the patch compartment could be a homeostatic response to increased dopamine release in the CPu, as kappa opioid receptor agonists have been shown to decrease dopamine release in the CPu (Margolis et al., 2003; Meshul and McGinty, 2000; Walker et al., 1987; You et al., 1999). In line with this theory is the observation that METH-induced stereotypy first occurs 40-60 min after METH treatment (see Fig. 2), a time point at which there is not significant induction of preprodynorphin mRNA expression by METH or AMPH (Adams et al., 2003; Horner and Keefe, 2006; Wang et al., 1995).
It is of note that in the current study, arc mRNA expression, which is typically homogeneous at 3h post-AMPH treatment (Gonzalez-Nicolini and McGinty, 2002) was patch-enhanced in the DL CPu, 3h after METH treatment. It is possible that the quadrant-based patch-matrix analysis utilized in our study unmasked regional alterations in patch-to-matrix arc expression that may not be detectable in the CPu as a whole. Alternatively, there may be subtle, region-specific patterns of arc expression induced by AMPH vs. METH after extended exposure. Regardless, a patch-enhanced pattern of arc expression in the DL CPu at 3h post-treatment could reflect a transition from a generalized patch-enhanced pattern of arc expression evident at shorter survival times to a restricted patch-enhanced pattern of arc expression within this region, as the stereotypic behavior becomes more intense in its inflexibility. However, it is not clear how this focused pattern of patch-enhanced arc expression in the DL CPu would serve as a homeostatic response to METH-induced overstimulation of striatal neurons and stereotypy. Additional studies are needed in order to determine the exact nature of the negative relationship between patch-enhanced arc expression and METH-induced stereotypy.
Striatal mu opioid receptor blockade and METH-induced gene expression and stereotypy
Striatal mu opioid receptor blockade attenuated METH-induced gene expression in the patch and matrix compartments in all four sub-regions of rostral CPu. The ability of mu opioid receptor blockade to attenuate METH-induced gene expression in the matrix compartment is unexpected, since mu opioid receptors are not significantly expressed in this region of CPu (Herkenham and Pert, 1981; Pert et al., 1976; Tempel and Zukin, 1987). The ability of CTAP to attenuate mRNA expression in the matrix compartment could reflect interactions between the patch and matrix compartments. The majority of medium spiny neurons in the CPu have dendritic arborizations that remain within their compartment of origin, but a portion of medium spiny neurons have dendritic arborizations that cross from one compartment into the other (Bolam et al., 1988; Walker et al., 1993). Thus, it is possible that dendrites from a portion of matrix neurons cross over into the patch and are influenced by CTAP-induced changes in the patch compartment.
Alternatively, striatal mu opioid receptor blockade may have reduced dopamine release in the CPu, leading to decreased activation of both compartments. Disinhibition of the GABAergic neurons of the patch compartment via mu opioid receptor blockade could inhibit dopaminergic cell firing in the substantia nigra pars compacta, resulting in decreased dopamine release in the CPu (Gonzalez-Nicolini et al., 2003; Ujike et al., 1989). The potential CTAP-induced decreases in METH-induced striatal dopamine release could also explain how mu opioid receptor blockade was able to reduce METH-induced stereotypy, as well as disrupt the correlation between patch-enhanced PD and arc mRNA expression and stereotypy. If mu opioid receptor blockade can reduce METH-induced dopamine release in the CPu, then the dopamine-driven homeostatic response of patch-enhanced PD expression by METH would be diminished in the presence of CTAP, leading to a reduction in METH-induced stereotypy and patch-enhanced PD expression. As for arc, there may still have been enough METH-induced dopamine release in the presence of CTAP to co-activate D1 and D2 receptors (which has been shown to be necessary for patch-enhanced IEG expression in the CPu) allowing for the maintenance of METH-induced patch-enhanced arc expression (Capper-Loup et al., 2002; LaHoste et al., 1993). As such, the disruption of the negative correlation between patch-enhanced arc mRNA expression and METH-induced stereotypy by CTAP was not due to a decrease in patch-enhanced arc mRNA expression, but the result of the reduction in cumulative stereotypy scores. These data indicate that patch-enhanced arc mRNA expression is independent of METH-induced stereotypy when mu opioid receptors are blocked. In support of this observation are previous studies that have demonstrated a dissociation can exist between patch-enhanced IEG expression in the CPu and the stereotypy induced by METH treatment, indicating that patch predominance in the CPu may not necessarily be related to repetitive behaviors (Glickstein and Schmauss, 2004). However, the specific effects of striatal mu opioid receptor blockade on METH-induced dopamine release in the CPu are not currently known, but it is important to that note METH-induced increases in extracellular dopamine may not be action potential-dependent (Nomikos et al., 1990) and mu opioid receptor blockade does not alter AMPH-induced dopamine release in the CPu (Schad et al., 1996). Clearly, additional studies are needed to address the potential modulatory role of mu opioid receptor activation in METH-induced gene expression and behavior.
Conclusions
In summary, our findings demonstrate that striatal mu opioid receptor activation contributes to METH-induced gene expression in both the patch and matrix compartments of the CPu, suggesting compartmental cross-talk or mu opioid receptor-mediated modulation of factors that contribute to gene expression in both regions. This study is also the first to demonstrate a negative correlation between a patch-enhanced pattern of PD and arc mRNA expression in the DL CPu and the overall expression of METH-induced stereotyped behavior, suggesting that patch-enhanced expression of these genes may be a homeostatic response to METH-induced overstimulation of the CPu, rather than the source of METH-induced stereotypy. Striatal mu opioid receptor blockade also reduced METH-induced stereotypy, as well as the correlation between patch-enhanced PD and arc expression and behavior, suggesting that mu opioid receptor activation may contribute to this relationship. Together, these data indicate that the mu opioid receptor system contributes to the systemic changes in basal ganglia function and organismal behavior that occur as a result of METH treatment.
Acknowledgments
This work was supported by a grant from the National Institute on Drug Abuse to KAH (DA025303). We thank Dr. Paul Frankel for his comments regarding this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams AC, Layer RT, McCabe T, Keefe KA. Effects of conanotoxins on L-3-4-dihydroxyphenylalanine-induced behavior and immediate early gene expression. Eur J Pharmacol. 2000;404:303–313. doi: 10.1016/s0014-2999(00)00640-3. [DOI] [PubMed] [Google Scholar]
- Adams DH, Hanson GR, Keefe KA. Differential effects of cocaine and methamphetamine on neurotensin/neuromedin N and preprotachykinin messenger RNA expression in unique regions of the striatum. Neuroscience. 2001;102:843–851. doi: 10.1016/s0306-4522(00)00530-3. [DOI] [PubMed] [Google Scholar]
- Adams DH, Hanson GR, Keefe KA. Distinct effects of methamphetamine and cocaine on preprodynorphin messenger RNA in rat striatal patch and matrix. J Neurochem. 2003;84:87–93. doi: 10.1046/j.1471-4159.2003.01507.x. [DOI] [PubMed] [Google Scholar]
- Bolam JP, Izzo PN, Graybiel AM. Cellular substrate of the histochemically defined striosome and matrix system of the caudate nucleus: a combined golgi and immunohistochemical study. Neuroscience. 1988;24:853–875. doi: 10.1016/0306-4522(88)90073-5. [DOI] [PubMed] [Google Scholar]
- Canales JJ, Graybiel AM. A measure of striatal function predicts motor stereotypy. Nat Neurosci. 2000;3:377–383. doi: 10.1038/73949. [DOI] [PubMed] [Google Scholar]
- Capper-Loup C, Canales JJ, Kadaba N, Graybiel AM. Concurrent activation of dopamine D1 and D2 receptors is required to evoke neural and behavioral phenotypes of cocaine sensitization. J Neurosci. 2002;22:6216–6227. doi: 10.1523/JNEUROSCI.22-14-06218.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civelli O, Douglass J, Goldstein A, Herbert E. Sequence and expression of the rat dynorphin gene. Proc Natl Acad Sci USA. 1985;82:4291–4295. doi: 10.1073/pnas.82.12.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole RL, Konradi C, Douglass J, Hyman SE. Neuronal adaptation to amphetamine and dopamine: molecular mechanisms of prodynorphin gene regulation in rat striatum. Neuron. 1995;14:813–823. doi: 10.1016/0896-6273(95)90225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creese I, Iverson SD. Blockage of amphetamine-induced motor stimulation and stereotypy in the adult rat following neonatal treatment with 6-hydroxydopamine. Brain Res. 1973;55:369–382. doi: 10.1016/0006-8993(73)90302-8. [DOI] [PubMed] [Google Scholar]
- Fosnaugh JS, Bhat RV, Yamagata K, Worley PF, Baraban JM. Activation of arc, a putatuve “effector” immediate early gene, by cocaine in rat brain. J Neurochem. 1995;64:2377–2380. doi: 10.1046/j.1471-4159.1995.64052377.x. [DOI] [PubMed] [Google Scholar]
- Frankel PS, Hoonakker AJ, Danaceau JP, Hanson GR. Mechanism of an exaggerated locomotor response to a low-dose challenge of methamphetamine. Pharmacol Biochem Behav. 2007;86:511–515. doi: 10.1016/j.pbb.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic: compartmentalization of corticostriatal input and striatonigral output systems. Nature. 1984;311:461–464. doi: 10.1038/311461a0. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic: striatal patch-matrix organization is related to cortical lamination. Science. 1989;246:385–388. doi: 10.1126/science.2799392. [DOI] [PubMed] [Google Scholar]
- Glickstein SB, Schmauss C. Focused stereotypies do not require enhanced activation of neurons in striosomes. J Comp Neurol. 2004;469:277–238. doi: 10.1002/cne.11000. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Nicolini MV, Berglind W, Cole KS, Keogh C, McGinty JF. Local mu and delta opioid receptors regulate amphetamine-induced behavior and neuropeptide mRNA in the striatum. Neuroscience. 2003;121:387–398. doi: 10.1016/s0306-4522(03)00488-3. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Nicolini V, McGinty JF. Gene expression profile from the striatum of amphetamine-treated rats: a cDNA array and in situ hybridization histochemical study. Gene Expression Patterns. 2002;1:193–198. doi: 10.1016/s1567-133x(02)00017-0. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Canales JJ. The neurobiology of repetitive behaviors: clues to the neurobiology of Tourette syndrome. Adv Neurol. 2000;85:123–131. [PubMed] [Google Scholar]
- Graybiel AM, Canales JJ, Capper-Loup C. Levodopa-induced dyskinesias and dopamine-dependent stereotypies: a new hypothesis. Trends Neurosci. 2000;23:S71–S77. doi: 10.1016/s1471-1931(00)00027-6. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Rauch SL. Toward a neurobiology of obsessive-compulsive disorder. Neuron. 2000;28:343–347. doi: 10.1016/s0896-6273(00)00113-6. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Pert CB. Mosaic distibution of opiate receptors, parafascilular projections and acetylcholinesterase in rat striatum. Nature. 1981;291:415–418. doi: 10.1038/291415a0. [DOI] [PubMed] [Google Scholar]
- Horner KA, Adams DH, Hanson GR, Keefe KA. Blockade of stimulant-induced preprodynoprhin mRNA expression in the rat striatal matrix by serotonin depletion. Neuroscience. 2005;131:67–77. doi: 10.1016/j.neuroscience.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Horner KA, Keefe KA. Regulation of psychostimulant-induced preprodynorphin, c-fos and zif/268 messenger RNA expression in the rat dorsal striatum by mu opioid receptor blockade. Eur J Pharmacol. 2006;532:61–73. doi: 10.1016/j.ejphar.2005.12.041. [DOI] [PubMed] [Google Scholar]
- Horner KA, Noble ES, Lauterbach EC. Differential regulation of prodynophin, c-fos, and serotonin transporter mRNA following withdrawal from a chronic, escalating dose regimen of D-amphetamine. Synapse. 2009;63:257–268. doi: 10.1002/syn.20606. [DOI] [PubMed] [Google Scholar]
- Keefe KA, Gerfen CR. D1-D2 dopamine receptor synergy in striatum: effects of intrastriatal infusions of dopamine agonistss and antagonists on immediate early gene expression. Neuroscience. 1995;66:903–913. doi: 10.1016/0306-4522(95)00024-d. [DOI] [PubMed] [Google Scholar]
- Kramer TH, Shook JE, Kzamierski W, Ayres EA, Wire WS, Hruby VJ, Burks TF. Novel peptidergic mu opioid antagonists: pharmacological characterization in vitro and in vivo. J Pharmacol Exp Ther. 1988;249:544–551. [PubMed] [Google Scholar]
- LaHoste GJ, Yu J, Marshall JF. Striatal Fos expression is indicative of dopamine D1/D2 synergism and receptor supersensitivity. Proc Natl Acad Sci USA. 1993;90:7451–7455. doi: 10.1073/pnas.90.16.7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyford G, Yamagata K, Kaufmann W, Barnes C, Sanders L, Copeland N, Gilbert D, Jenkins N, Lanahan A, Worley PF. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14:433–445. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Hjelmstad GO, Bonci A, Fields HL. k-opioid agonists directly inhibit midbrain dopaminergic neurons. J Neurosci. 2003;23:9981–9986. doi: 10.1523/JNEUROSCI.23-31-09981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshul CK, McGinty JF. Kappa opioid receptor immunoreactivity in the nucleus accembens and caudate-putamen is primarily associated with synpatic vesicles in axons. Neuroscience. 2000;96:91–99. doi: 10.1016/s0306-4522(99)90481-5. [DOI] [PubMed] [Google Scholar]
- Mildbrandt J. A nerve growth-factor-induced gene encodes a possible transcriptional regulatory factor. Science. 1987;248:797–799. doi: 10.1126/science.3672127. [DOI] [PubMed] [Google Scholar]
- Moratalla R, Robertson HA, Graybiel AM. Dynamic regulation of NGFI-A (zif268, egr1) gene exprssion in the striatum. J Neurosci. 1992;12:2609–2622. doi: 10.1523/JNEUROSCI.12-07-02609.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomikos GG, Damsma G, Wenkstern D, Fibiger HC. In vivo characterization of locally applied dopamine uptake inhibitors by striatal microdialysis. Synapse. 1990;6:106–112. doi: 10.1002/syn.890060113. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Elsevier Academic Press; 2005. [Google Scholar]
- Pert CB, Kuhar M, Snyder SH. Opiate receptor: autoradiographic localization in the rat brain. Proc Natl Acad Sci USA. 1976;73:3729–3733. doi: 10.1073/pnas.73.10.3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presti ME, Lewis MH. Striatal opioid peptide content in an animal model of spontaneous stereotypic behavior. Behav Brain Res. 2005;157:363–368. doi: 10.1016/j.bbr.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Ragsdale CW, Jr, Graybiel AM. Fibers from the basolateral amygdala selectively innervate the striosomes in the caudate nucleus of the cat. J Comp Neurol. 1988;269:506–522. doi: 10.1002/cne.902690404. [DOI] [PubMed] [Google Scholar]
- Schad CA, Justice JB, Jr, Holtzman SG. Differential effects of delta- and mu-opioid receptor antagonists on the amphetamine-induced increase in extracellular dopamine in striatum and nucleus accumbens. J Neurochem. 1996;67:2292–2299. doi: 10.1046/j.1471-4159.1996.67062292.x. [DOI] [PubMed] [Google Scholar]
- Steward O, Wallace CS, Lyford G, Worley PF. Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron. 1998;21:741–751. doi: 10.1016/s0896-6273(00)80591-7. [DOI] [PubMed] [Google Scholar]
- Steward O, Worley PF. Selective targeting of newly synthesized Arc mRNA to actived synapses requires NMDA receptor activation. Neuron. 2001;30:227–240. doi: 10.1016/s0896-6273(01)00275-6. [DOI] [PubMed] [Google Scholar]
- Tan A, Moratalla R, Lyford G, Worley PF, Graybiel AM. The activity-regulated cytoskeletal-associated protein Arc is expressed in different striosome-matrix patterns following exposure to amphetamine and cocaine. J Neurochem. 2000;74:2074–2078. doi: 10.1046/j.1471-4159.2000.0742074.x. [DOI] [PubMed] [Google Scholar]
- Tempel A, Zukin RS. Neuroanatomical patterns of the mu, delta and kappa opioid receptors of the rat brain as determined by quantitative in vitro autoradiography. Proc Natl Acad Sci USA. 1987;43:4308–4312. doi: 10.1073/pnas.84.12.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujike H, Onoue T, Akiyama K, Hamamura T, Otsuki S. Effects of selective D-1 and D-2 dopamine antagonists on development of methamphetamine-induced behavioral sensitization. Psychopharmacology. 1989;98:89–92. doi: 10.1007/BF00442011. [DOI] [PubMed] [Google Scholar]
- Walker JM, Thompson LA, Frascella J, Friederich MW. Opposite effects of mu and kappa opiate on the firing-rate of dopamine cell in the substantia nigra of the rat. Eur J Pharmacol. 1987;134:53–59. doi: 10.1016/0014-2999(87)90130-0. [DOI] [PubMed] [Google Scholar]
- Walker RH, Arbuthnott GW, Baughman RW, Graybiel AM. Dendritic domains of medium spiny neurons in the primate striatum: relationship to striosomal borders. J Comp Neurol. 1993;337:614–628. doi: 10.1002/cne.903370407. [DOI] [PubMed] [Google Scholar]
- Wang JQ, McGinty JF. Dose-dependent alterations in zif/268 and preprodynorphin mRNA exprsesion induced by amphetamine and methamphetamine in rat forebrain. J Pharmacol Exp Ther. 1995;273:909–917. [PubMed] [Google Scholar]
- Wang JQ, Smith AJ, McGinty JF. A single injection of amphetamine or methamphetamine induces dynamic alterations in c-fos, zif/268 and preprodynorphin messenger RNA expression in the rat forebrain. Neuroscience. 1995;68:83–95. doi: 10.1016/0306-4522(95)00100-w. [DOI] [PubMed] [Google Scholar]
- Woo SK, Hitzemann RJ, Loh HH. Specific opioid-amphetamine interactions in the caudate putamen. Psychopharmacology. 1985;85:371–376. doi: 10.1007/BF00428204. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- You ZB, Herrera-Marchitz M, Terenius L. Modulation of neurotransmitter release in the basal ganglia of the rat brain by dynorphin peptides. J Pharmacol Exp Ther. 1999;290:1307–1315. [PubMed] [Google Scholar]