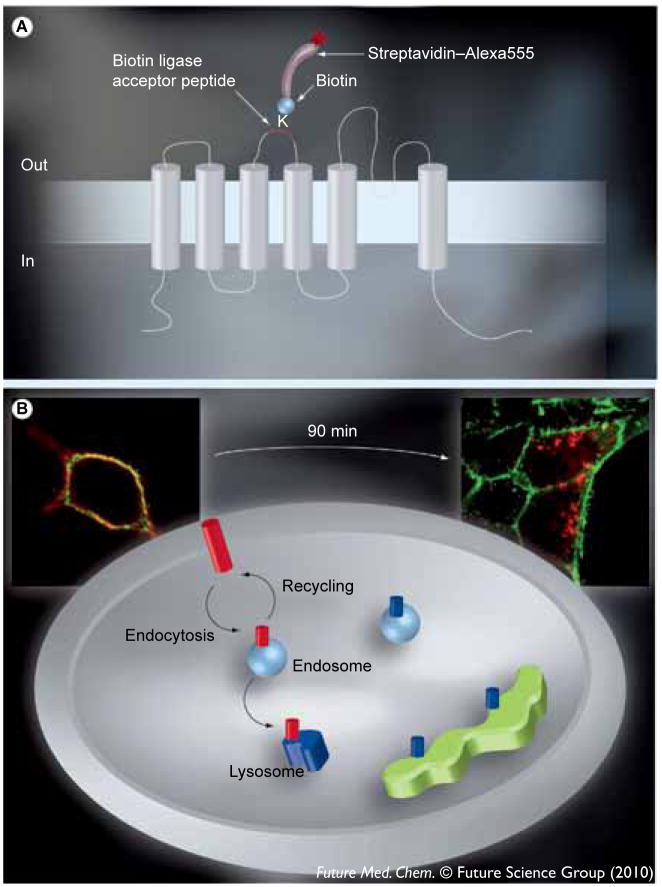
Figure 1.
(A) The architecture of KCa3.1 relative to the plasma membrane and the position of the 17 amino acid Bacillus licheniformis β-lactamase (BLAP) epitope tag between the third and fourth transmembrane domains. KCa3.1 is a six transmembrane domain K+ channel in which the N- and C-termini are cytoplasmic. The lysine within the BLAP sequence is specifically labeled with biotin via the co-expressed BirA-KDEL in the endoplasmic reticulum. Plasma membrane-expressed channels are then specifically labeled with streptavidin–Alexa555 such that only plasma membrane localized channels are tagged with fluorophore. (B) The labeling of KCa3.1 and the plasma membrane with unique fluorophores. Following labeling of plasma membrane channels with streptavidin–Alexa555 (red) and the plasma membrane itself with WGA–Alexa488 (green) we can discriminate between plasma membrane-localized KCa3.1 and channels in either endosomes or lysosomes, as illustrated. Channels within the biosynthetic pathway are biotinylated, but not fluorescently tagged (shown in blue), and therefore not recognized in this assay. High-resolution images of individual cells are shown to further illustrate this protocol. As shown at time 0, KCa3.1 is localized exclusively to the plasma membrane. This colocalization is shown as yellow. By contrast, after 90 min at 37°C, KCa3.1 has been endocytosed (red endosomes). By examining the loss of colocalization we can determine the amount of endocytosis and whether this is affected by small molecules. Cellular images were captured with an Olympus IX-81 epifluorescence microscope equipped with a 60× oil objective. Multiple z-planes were captured, the images deconvolved and shown as a projection image.