Table 2.
[4 + 2] Cycloadditions of Conjugated Enynamides with Alkynes
| entry | ynamide | conditionsa | indoline | yield (%)b |
|---|---|---|---|---|

| ||||
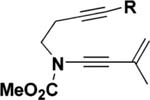
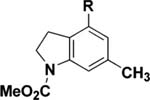
| 1 | 25 (R = Me) | 110 °C, 8 h | 30 | 96 |
| 2 | 25 (R = Me) | 110 °C, 8 hc | 30 | 80 |
| 3 | 25 (R = Me) | 110 °C, 8 hd | 30 | 72 |
| 4 | 25 (R = Me) | 0 °C to rt, 3 he | 30 | 78 |
| 5 | 26 (R = t-Bu) | 110 °C, 8 h | 31 | 91 |
|
|
|||
| 6 | 27 (R = SiMe3) | 180 °C, 16 h | 32 | 86 |
| 7 | 28 (R = CO2Me) | 110 °C, 30 h | 33 | 95 |
| 8 | 29 R = C≡CC(Me)2OSi(i-Pr)3) | 110 °C, 30 h | 34 | 84 |
Cycloadditions performed in toluene (0.05 M) with 1 equiv of BHT unless otherwise indicated.
Isolated yields.
Reaction in the absence of BHT.
Reaction in TFE (0.05 M) without BHT.
Reaction in the presence of 2.5 equiv of Me2AlCl in CH2C12 (0.05 M) without BHT.
