Abstract
Background and objectives: Our aim was to evaluate the growth-promoting effect of growth hormone (GH) treatment in infants with chronic renal failure (CRF) and persistent growth retardation despite adequate nutritional and metabolic management.
Design, setting, participants, & measurements: The study design included randomized, parallel groups in an open, multicenter trial comparing GH (0.33 mg/kg per wk) with nontreatment with GH during 12 months. Sixteen infants who had growth retardation, were aged 12 ± 3 months, had CRF (GFR ≤60 ml/min per 1.73 m2), and had adequate nutritional intake and good metabolic control were recruited from eight pediatric nephrology departments from Spain and Portugal. Main outcome measures were body length, body weight, bone age, biochemical and hormonal analyses, renal function, bone mass, and adverse effects.
Results: Length gain in infants who were treated with GH was statistically greater (P < 0.05) than that of nontreated children (14.5 versus 9.5 cm/yr; SD score 1.43 versus −0.11). The GH-induced stimulation of growth was associated with no undesirable effects on bone maturation, renal failure progression, or metabolic control. In addition, GH treatment improved forearm bone mass and increased serum concentrations of total and free IGF-I and IGF-binding protein 3 (IGFBP-3), whereas IGF-II, IGFBP-1, IGFBP-2, GH-binding protein, ghrelin, and leptin were not modified.
Conclusions: Infants with CRF and growth retardation despite good metabolic and nutritional control benefit from GH treatment without adverse effects during 12 months of therapy.
Growth retardation is still an important manifestation of pediatric chronic renal failure (CRF). The North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) 2008 annual report showed that the mean height of 7037 pediatric patients with CRF was −1.44 SD score (SDS) at the moment of study entry, with >35% of patients being less than the third percentile for height (1). Although growth impairment in CRF is of multifactorial origin, low caloric intake and resistance to growth hormone (GH) are major pathogenic factors (2,3).
Patients with advanced CRF have anorexia, and it is generally agreed that growth in the infantile period is mostly dependent on nutrition (4). Thus, aggressive nutritional management is recommended for infants who have CRF to achieve normal growth and development (5). Kari et al. (6) reported good growth and even catch-up growth in infants who had CRF and received forced-feeding. Parekh et al. (7) reported that nutritional support with sodium and water supplementation can maintain or improve the growth of children with polyuric, salt-wasting CRF. Although acknowledging the need for optimal nutritional management, some studies indicated that height deficit frequently cannot be overcome despite aggressive supplemental feeding (8–10).
This study was undertaken to determine whether longitudinal growth remains unsatisfactory in some well-nourished infants with CRF and whether therapy with GH could improve growth retardation in this specific group of patients. This study was also designed to provide information on nutritional biochemical indices and bone mass in these infants.
Materials and Methods
Patients
The study population met the following inclusion criteria: (1) Chronological age 12 ± 3 months; (2) GFR ≤60 ml/min per 1.73 m2, as calculated by the Schwartz formula (11); (3) length below −2 SDS for the same chronological age and growth velocity below the 50th percentile (12); (4) conservative treatment or long-term peritoneal dialysis; (5) euthyroid status; and (6) nutritional intake, assessed by dietary records, providing a daily amount of at least 80% of recommended dietary allowances for calories and 10% of calories from high biologic value proteins. Each hospital's dietetics and nutrition department was involved in the care and follow-up of these patients, and, following their indications and close supervision, energy supplements and enteral feeds were used when necessary. Exclusion criteria were hormonal, neurologic, osseous, genetic, or nephrologic conditions, other than CRF, that are known to interfere adversely with growth, as well as suspected allergy to the trial product or treatment with corticosteroids and inadequate metabolic control of CRF as shown by severe hyperparathyroidism, sustained metabolic acidosis, or sodium or water deficits.
Sixteen patients (13 boys) from eight centers in Spain and Portugal were enrolled in the study. Sample size was based on the achievement of a statistically significant difference in the increase of growth rate between the two study groups of at least 4.8 cm/yr (SD 2.6 cm/yr) (10) with an error α < 0.05 and an error β < 0.15 (power 85%). Patients were randomly classified into two groups of eight patients each: Untreated and treated with GH. One patient from each group was withdrawn from the study because of poor clinical and metabolic control. Causes of CRF were renal dysplasia/hypoplasia (11 cases), obstructive uropathy (three cases), hemolytic uremic syndrome (one case), and acute tubular necrosis (one case). Two patients and one patients in the untreated and GH treated groups, respectively, were on long-term peritoneal dialysis. Forced feeding by nasogastric tube or gastrostomy was used for seven infants; four were assigned to the GH treated group, and three were assigned to the untreated group.
Study Design
This was a multicenter, prospective, controlled, randomized, open clinical trial. Patients were stratified according to their gestational age (≤ or >37 weeks) and modality of CRF treatment (conservative or dialysis) and randomly assigned in a 1:1 distribution between groups. The process of randomization was centralized at Novo Nordisk A/S (Bagsvaerd, Denmark).
Treatment
GH (Norditropin SimpleXx; Novo Nordisk A/S) at 0.33 mg/kg per wk was injected daily at bedtime by subcutaneous route for 12 months. Efficacy of treatment was assessed by the evolution of the length SDS and growth velocity SDS. Safety was assessed by the effects of treatment on bone maturation, blood count, kidney function, glycosylated hemoglobin (HbA1c), insulin and glucose levels, intact parathyroid hormone concentrations, thyroid function, and the incidence of adverse reactions. Vials accountability and changes in serum IGF-I and IGF-binding protein 3 (IGFBP-3) levels were used as indicators of GH treatment compliance. The influence of GH on other hormonal and nutritional parameters was assessed by measurement of the serum concentrations of IGFBP-1 and -2, GH-binding protein (GHBP), leptin, and ghrelin. The potential effects of GH therapy on bone mass were also estimated.
Auxologic Data
The following parameters were measured: Every 3 months (visits 0, 3, 6, 9, and 12 months), medical history and physical exploration including BP, body length, body weight, head circumference, brachial perimeter, forearm length, and serum hematology and biochemistry; every 6 months (visits 0, 6, and 12 months), HbA1c and serum ferritin; and every 12 months (visits 0 and 12 months), fasting insulin, thyroid-stimulating hormone, free T4, bone age, and bone mass.
Auxologic data were normalized according to age and gender for the Spanish population (12). Bone maturation advance was assessed by the bone age–chronological age ratio.
Variable Measurements
All analytical determinations were performed in the laboratory of each participating center by automated procedures, except for total and free IGF-I, IGF-II, IGFBP-1, IGFBP-2, IGFBP-3, GHBP, leptin, and ghrelin, which were centralized in the Pediatric Endocrinology laboratory of the Hospital Infantil Universitario Niño Jesús (Madrid, Spain) and expressed in SDS by using reference values for healthy Spanish children (13,14). Bone age measured by using an x-ray of the left ankle (15) was centralized in the same hospital and performed by two investigators who were blinded for the study. Measurement of bone mass in the radius by dual-energy x-ray absorptiometry (QRD-4500W; Hologic, Waltham, MA) was centralized in the Mineral and Bone Metabolism Department of the Hospital Universitario Central de Asturias (Oviedo, Spain) and performed by one investigator (C.G.), who was blinded to the patients' assignment.
Total IGF-I and IGF-II levels were determined by RIA (Nichols Laboratories, Los Angeles, CA; and Endocrine Sciences, Calabasas Hill, CA, respectively) after acid-ethanol extraction of serum. IGFBP-3 levels were measured by RIA (Nichols Laboratories). Intra- and interassay coefficients of variation were 4.9, 1.8, and 3.6% and 8.9, 10.6, and 6.1% for IGF-I, IGF-II and IGFBP-3, respectively. IGFBP-1 was determined by ELISA (Medix Biochemica, Kauniainen, Finland). Intra- and interassay coefficients of variation were 4.6 and 9.8% for IGFBP-1. GHBP was determined by a monoclonal assay (13). Intra- and interassay coefficients of variation were 5.6 and 9.5%. Leptin and ghrelin levels were measured as previously reported (13). IGFBP-2 and free IGF-I were measured by RIA (Diagnostic Systems Laboratories, Webster, TX). Intra- and interassay variation coefficients were 5.7 and 7.2% for IGFBP-2 and 6.2 and 7.3% for free IGF-I.
Statistical Analysis
Unless otherwise specified, quantitative variables are given as mean ± SEM. The Kolmogorov-Smirnov test was used to check for normality in the continuous variables. Baseline quantitative variables were compared for homogeneity using a t test for independent groups (normal distribution) or by a Mann-Whitney U test (nonparametric distribution). Categorical variables were compared by the Fisher exact test or the χ2 test. Efficacy variables were analyzed by ANOVA with the treatment group as the main factor. All post hoc comparisons were performed using t tests with Bonferroni adjustment. ANOVA with repeated measures was performed to compare results from the various study visits; the cumulative gain in height and weight SDS was also compared by analysis of covariance. A two-sided α error of 5% was considered for all comparison analyses.
The study agreed with the Declaration of Helsinki and was approved by the ethics committee of clinical investigation of each participating center. The Health Ministries of Spain and Portugal approved the protocol and the informed consent form, which was signed by the infants' parents or guardians.
Results
Baseline Characteristics
There were no baseline differences between the two groups of patients (Tables 1 and 2). Both groups had a similar degree of growth retardation and renal function reduction. Head and brachial circumferences and forearm length were also similar. Serum albumin concentrations were normal in both groups, although they were significantly (P < 0.05) higher in the untreated group (44.5 ± 1.5 versus 38.3 ± 2.4 g/L).
Table 1.
Baseline auxologic variables in both groups of patients
| Variable | Untreated Group (n = 7) | GH-Treated Group (n = 7) |
|---|---|---|
| Gestational age (weeks) | 37.70 ± 0.81 | 37.00 ± 1.05 |
| Mother's height (cm) | 153.90 ± 1.75 | 157.50 ± 2.35 |
| Father's height (cm) | 173.60 ± 1.78 | 173.30 ± 1.88 |
| Weight at birth (kg) | 2.74 ± 0.14 | 2.67 ± 0.18 |
| Length at birth (cm) | 46.50 ± 0.85 | 44.30 ± 2.32 |
| Chronological age (months) | 14.00 ± 0.65 | 14.40 ± 0.84 |
| Bone age (months) | 9.40 ± 1.07 | 9.20 ± 0.49 |
| Bone age–chronological age ratio | 0.67 ± 0.08 | 0.69 ± 0.06 |
| Length SDS | −3.13 ± 0.50 | −3.00 ± 0.30 |
| Weight SDS | −2.03 ± 0.41 | −2.68 ± 0.33 |
| Growth velocity SDS | −2.76 ± 0.28 | −3.81 ± 0.56 |
Data are means ± SEM. There were no statistically significant differences between groups for any of the analyzed variables.
Table 2.
Baseline serum biochemical and hormonal variables and GFR, estimated by the endogenous creatinine clearance calculated by the Schwartz formula (11), in both groups of patients
| Variable | Untreated Group (n = 7) | GH-Treated Group (n = 7) |
|---|---|---|
| Creatinine (μmol/L) | 174.0 ± 49.7 | 281.2 ± 104.4 |
| GFR (ml/min per 1.73 m2) | 27.8 ± 7.9 | 23.0 ± 6.3 |
| BUN (mmol/L) | 10.5 ± 3.0 | 25.9 ± 7.8 |
| Calcium (mmol/L) | 2.36 ± 0.19 | 2.26 ± 0.35 |
| Phosphate (mmol/L) | 1.95 ± 0.28 | 1.69 ± 0.19 |
| Intact PTH (pg/ml) | 221.1 ± 110.2 | 333.1 ± 179.8 |
| Bicarbonate (mmol/L) | 24.3 ± 1.2 | 22.4 ± 0.9 |
| Insulin (pmol/L) | 63.4 ± 30.1 | 70.7 ± 30.1 |
| Total IGF-I (ng/ml) | 121.3 ± 23.3 | 89.4 ± 25.6 |
| Total IGF-I SDS | −0.73 ± 0.12 | −0.86 ± 0.13 |
| Free IGF-I (ng/ml) | 0.34 ± 0.05 | 0.39 ± 0.10 |
| Free IGF-I SDS | −0.06 ± 0.41 | 0.50 ± 0.76 |
| IGF-II (ng/ml) | 684.0 ± 75.2 | 624.4 ± 79.4 |
| IGF-II SDS | 1.04 ± 0.63 | 0.70 ± 0.64 |
| IGFBP-1 (ng/ml) | 127.0 ± 20.3 | 98.7 ± 23.9 |
| IGFBP-1 SDS | 6.87 ± 1.19 | 5.19 ± 1.41 |
| IGFBP-2 (ng/ml) | 3207.9 ± 578.9 | 3012.4 ± 495.9 |
| IGFBP-2 SDS | 19.24 ± 3.89 | 18.15 ± 3.04 |
| IGFBP-3 (μg/ml) | 3.41 ± 0.51 | 2.94 ± 0.37 |
| IGFBP-3 SDS | 0.08 ± 0.47 | −0.26 ± 0.32 |
| GHBP (pmol/L) | 474.1 ± 161.1 | 546.1 ± 127.1 |
| GHBP SDS | −1.42 ± 0.36 | −1.26 ± 0.28 |
| Ghrelin (pg/ml) | 1606.3 ± 239.0 | 1556.4 ± 239.0 |
| Ghrelin SDS | 0.85 ± 0.91 | 0.81 ± 0.54 |
| Leptin (ng/ml) | 11.60 ± 3.64 | 11.50 ± 5.39 |
| Leptin SDS | 3.77 ± 2.15 | 3.77 ± 3.18 |
Data are means ± SEM. There were no statistically significant differences between groups for any of the analyzed variables. BUN, blood urea nitrogen; PTH, parathyroid hormone.
Growth
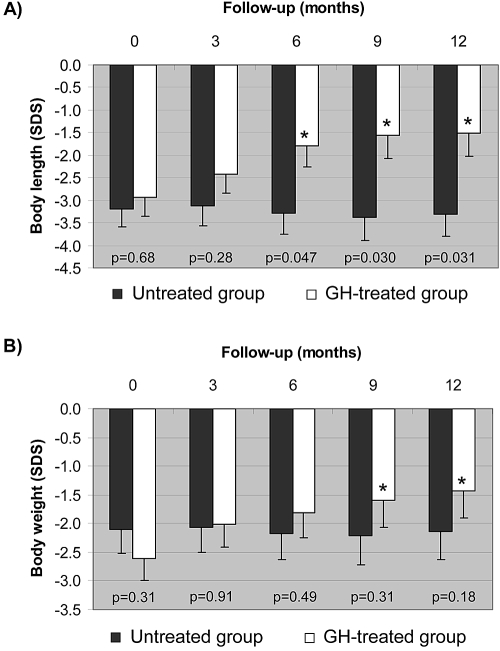
Body length SDS increased throughout the study in the GH-treated group only (Figure 1A). After 12 months, the GH-treated group gained 14.5 ± 1.2 cm and 1.4 ± 0.3 SDS versus 9.5 ± 1.1 cm and −0.1 ± 0.3 SDS in the untreated group (P = 0.024 and P = 0.031, respectively). Length SDS became different between the two groups from the 6-month follow-up onward (Figure 1A). Similarly, weight SDS of GH-treated patients became greater than that of untreated infants from the 9-month follow-up visit onward (Figure 1B). Head circumference increased in both groups, from 44.9 ± 0.8 to 47.8 ± 0.6 cm (P < 0.001) in the GH-treated group and from 45.3 ± 0.8 to 47.5 ± 0.6 cm (P < 0.001) in the untreated group, without significant differences between groups. There was no significant increment and no differences for brachial circumference and forearm length.
Figure 1.
(A and B) Evolution of body length (A) and body weight (B) during the 1-year study period in the two groups of infants with CRF. ■, Untreated group; □, GH-treated group. Data are means ± SEM of SDS for length. P values of intergroup comparisons at that particular time point. *Intragroup significant change compared with the corresponding baseline (Bonferroni adjusted P < 0.05).
Bone Mass
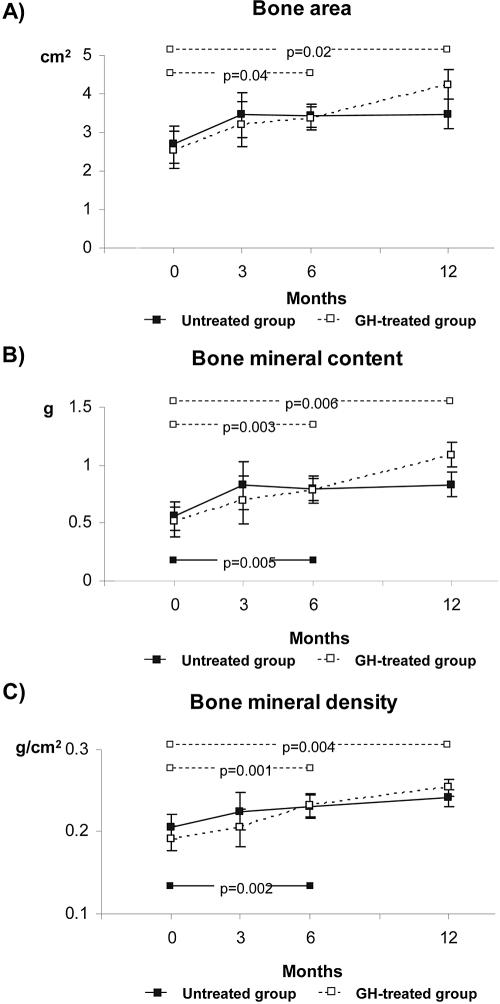
Bone area, bone mineral content, and bone mineral density increased from the 6-month visit onward in the GH-treated group (Figure 2). In the untreated group, bone mineral content and bone mineral density became higher than baseline at the 6-month visit, but the difference did not persist at the 12-month visit. There were no significant differences between groups at any study visit.
Figure 2.
Dual-energy x-ray absorptiometry results performed on total radius in both groups of patients. (A) Bone area (cm2). (B) Bone mineral content (g). (C) Bone mineral density (g/cm2). Data are means ± SEM. P values of intragroup comparisons at that particular time point. Solid and dashed lines and intragroup P values symbolize the untreated and the GH-treated groups, respectively.
Hormonal Determinations
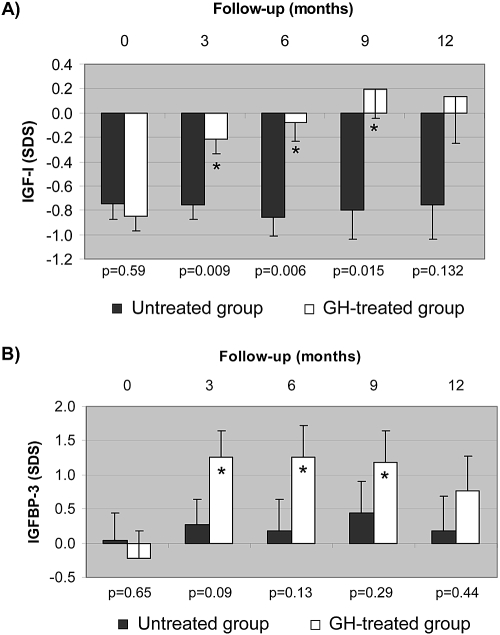
Total IGF-I SDS increased significantly after 3 months of GH treatment (from −0.85 ± 0.13 to −0.22 ± 0.12; P < 0.05) and remained so throughout the study (−0.08 ± 0.16, 0.20 ± 0.24, and 0.14 ± 0.38 at months 6, 9, and 12, respectively), whereas it did not change in the untreated group (−0.75 ± 0.13, −0.75 ± 0.12, −0.86 ± 0.16, −0.79 ± 0.24, and −0.75 ± 0.38 at months 0, 3, 6, 9, and 12; Figure 3). Free IGF-I SDS increased significantly after 9 and 12 months of GH treatment versus baseline (0.64 ± 0.52, 4.65 ± 1.07, 3.50 ± 0.93, 3.47 ± 0.81, and 3.28 ± 0.72 at months 0, 3, 6, 9, and 12). IGFBP-3 SDS increased significantly until month 9 (P < 0.05) with GH treatment from −0.22 ± 0.40 at baseline to 1.26 ± 0.38, 1.26 ± 0.46, 1.18 ± 0.47, and 0.77 ± 0.51 at months 3, 6, 9, and 12 of the study, respectively, whereas it did not change in the untreated group (0.04 ± 0.40, 0.27 ± 0.38, 0.19 ± 0.46, 0.44 ± 0.47, and 0.18 ± 0.51, respectively). There were no differences in SDS IGFBP-1 between both groups in basal and final visits; however, at months 3, 6, and 9 of the study, levels were significantly higher in the control group. No consistent variations throughout the study or differences between the groups were found for serum IGF-II, IGFBP-2, GHBP, ghrelin, or leptin (data not shown).
Figure 3.
(A and B) Evolution of IGF-I (A) and IGFBP-3 (B) during the 1-year study period in the two groups of infants with CRF. ■, Untreated group; □, GH-treated group. Data are means ± SEM of SDS for reference values. P values of intergroup comparisons at that particular time point. *Intragroup significant change compared with the corresponding baseline (Bonferroni adjusted P < 0.05).
Safety
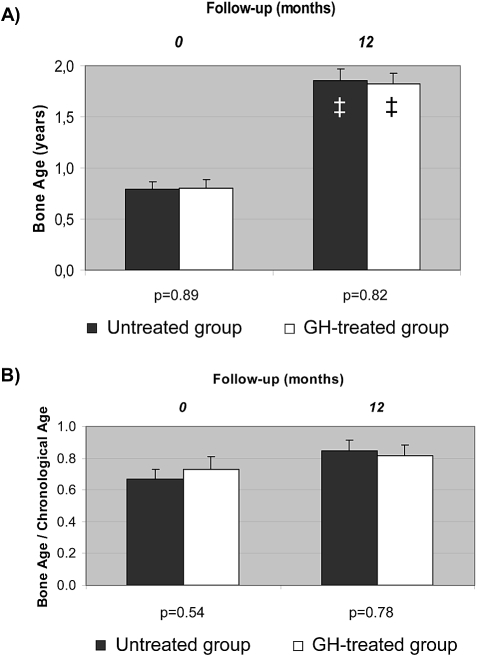
Bone age advanced similarly in both groups throughout the study: 0.98 ± 0.10 and 0.98 ± 0.12 years in GH-treated and untreated infants, respectively (Figure 4). Basal and final bone ages and bone age–chronological age ratios were not different between groups.
Figure 4.
(A and B) Basal and final bone age (A) and bone age–chronological age ratio (B) in both groups of patients. ■, Untreated group; □, GH-treated group. Data are means ± SEM. ‡Intragroup significant change compared with the corresponding baseline (Bonferroni adjusted P < 0.001).
BP, hemoglobin, leukocyte and platelet counts, serum concentrations of sodium, bicarbonate, total proteins, albumin, transaminases, fasting glucose, HbA1c, insulin, T4, thyroid-stimulating hormone, ferritin, cholesterol, and triglycerides remained within the normal range throughout the study without differences between groups. Serum concentrations of calcium, phosphate, 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D, and parathyroid hormone were similar in both groups and did not change throughout the study.
There were 29 adverse events, nine in the GH-treated group and 20 in the untreated group (P = 0.065). None was considered to have a possible relationship to the study drug. Adverse events that were considered to be mild or moderate were acute respiratory infection, acute media otitis, chickenpox, megaloerythema, abdominal pain, and acute gastroenteritis. There were six serious adverse events in the GH-treated group and five in the untreated group; in both groups, urinary tract infections and surgical procedures and one case of peritonitis in the control group. These serious adverse events caused a total of 43 and 29 days of hospitalization in the GH and control groups, respectively.
Discussion
This study shows that some infants with CRF do not grow well despite being well-nourished and having an adequate control of factors that are known to impair growth, such as anemia, metabolic acidosis, severe renal osteodystrophy, and water and salt deficiencies. This finding does not contradict former reports (6,7,16) indicating that optimization of nutrition in infants with CRF accelerates longitudinal growth velocity in these patients when considered as a group and even normalizes body length in some of them; however, the data presented here clearly show that a positive growth response to forced feeding does not always occur, and some infants with CRF and good nutritional and metabolic control continue to have growth retardation. Our study indicates that the majority of infants with CRF are able to exhibit adequate growth or catch-up growth with strict and careful nutritional support, an observation supported by the fact that many pediatric hospitals were needed just to fulfill the number of patients required in the trial.
In this particular group of patients, administration of GH for 1 year markedly improved longitudinal growth so that body length SDS significantly increased with respect to baseline values and to that of the untreated group. Length and weight SDS and other nutritional indexes did not change in the group of children who were not treated with GH, indicating that the nutritional management precluded the aggravation of growth failure. In concordance with former studies of older children (17,18), the growth-promoting effect of GH was not associated with exaggerated advancement of bone maturation, worsening of renal function, more difficult metabolic control, or higher incidence of adverse effects. A retrospective study recently showed improvement of height SDS in a group of infants who had CRF and were treated with 0.35 mg/kg per wk GH for 2 years (19).
Somatotropin is approved for the treatment of growth failure in children with CRF in United States, Europe, Japan, and Australia, although use of GH in this population remains low (1,18). Some practitioners and families are reluctant to initiate GH therapy in infants, and even in some countries, such as Spain, Portugal, and France, legal regulations preclude GH treatment in patients who have CRF and are younger than 2 years. Our study supports early treatment with GH in infants who have CRF and continue to have growth retardation after achieving good clinical, metabolic, and nutritional control. GH can be safely administered and improve growth retardation in a period of life when growth velocity is extremely high in normal conditions, and the change in height of children with CRF follows a typical pattern that aggravates growth deficit (20).
Baseline concentrations of circulating IGF-I within the normal range in the two groups of patients reflect the adequate nutritional status of infants included in the trial. The elevation of serum IGF-I and IGFBP-3 values in the group of children who were treated with GH confirms the good compliance with treatment. Baseline serum IGFBP-1 and IGFBP-2 concentrations were markedly increased in both groups of infants. Elevation of these IGFBP fractions have been reported in pediatric patients with CRF (21), although not specifically in infants. Circulating levels of insulin keep an inverse relationship with those of IGFBP-1 and IGFBP-2 in children with nutritional disorders (22,23). Thus, the association of high concentrations of IGFBP-1 and IGFBP-2 with normal values of serum insulin suggests that the status of insulin resistance reported in children with CRF (24,25) is present in well-nourished infants with CRF and is not modified by GH therapy.
Both groups of infants with CRF had hyperleptinemia and normal serum concentrations of ghrelin. Former studies found high serum leptin and a negative but weak correlation between energy intake and serum leptin concentrations in children in various stages of CRF (26,27). Other studies did not demonstrate high serum leptin values in children with CRF (28). Little is known about the circulating concentrations of ghrelin in pediatric patients with CRF. Normal (29) and elevated (28,30,31) plasma total ghrelin values have been found in small series of children who had CRF and were on long-term dialysis. The interrelation among leptin, ghrelin, and the control of appetite in individuals with CRF is still unclear. Ghrelin is orexigenic, but measurement of total ghrelin includes nonacylated and acylated (active) ghrelin. It is not the plasma level of the acylated, active form of ghrelin that is increased in uremia but instead the level of nonacylated ghrelin (32). High plasma levels of nonacylated ghrelin inhibit appetite in mice (33). Thus, accumulation of nonacylated ghrelin and leptin might be pathogenic factors of the anorexia of CRF. On the contrary, elevation of the circulating concentrations of these peptides might be an epiphenomenon that results from the reduced GFR. In our study, mean leptin and ghrelin values remained steady over time and did not change during GH therapy. Patel et al. (34) reported that GH treatment did not modify serum leptin levels in 11 prepubertal children with CRF. Our study confirms these results and shows for the first time that circulating values of ghrelin are not influenced by GH therapy in children with CRF. In agreement with the lack of effect of GH administration on the levels of leptin and ghrelin, experimental studies have shown that exogenous GH accelerates growth rate in individuals with CRF but does not modify appetite (35,36).
Data on bone composition in infants with CRF and the effect of GH are not available in the literature. In small series of older prepubertal children with CRF, bone mineral density of lumbar spine increased (37) or remained unchanged (38) with GH therapy. In our study, the growth-promoting effect of GH was initially accompanied by an increase in bone area and bone mineral content that reflected the stimulus of GH on longitudinal growth. After 12 months of treatment, a 39% improvement of radius mineral density in comparison with baseline values in the GH-treated group indicated a beneficial effect of GH on bone mineralization.
Conclusions
This study shows that a minority of infants with CRF do not grow well despite good nutrition and adequate metabolic and clinical control. These patients respond to GH treatment with a significant improvement in body length without adverse effects. In this particular group of infants, GH treatment should be initiated.
Disclosures
None.
Acknowledgments
The rest of recruiting investigators were Mercedes Navarro (Hospital Universitario Infantil La Paz, Madrid, Spain) and Manuel Peña (Hospital Universitario Materno Infantil. Complejo Hospitalario Carlos Haya, Málaga, Spain). The following are the nonrecruiting investigators who participated in the screening of patients: Ramón Areses (Hospital Donostia, San Sebastián, Spain), Alfonso Bao (Hospital Xeral Cíes, Vigo, Spain), Rafael Bedoya (Hospital Infantil, Ciudad Sanitaria Virgen del Rocío, Sevilla, Spain), Miguel García-Fuentes (Hospital Universitario Marqués de Valdecilla, Santander, Spain), Leopoldo García (Hospital Sant Joan de Déu, Barcelona, Spain), Víctor García-Nieto (Hospital Universitario Nuestra Señora de Candelaria, Santa Cruz de Tenerife, Spain), Emilia Hidalgo (Hospital Materno Infantil, Badajoz, Spain), César Loris (Hospital Universitario Infantil Miguel Servet, Zaragoza, Spain), Inmaculada Nadal (Hospital Virgen del Camino, Pamplona, Spain), Alfredo Vallo (Hospital de Cruces, Baracaldo, Spain), Mercedes Vázquez-Martul and José Luis Ecija (Hospital Niño Jesús, Madrid, Spain), and Margarida Almeida (Clínica Universitária de Pediatria do Hospital de Santa María, Lisboa, Portugal).
We thank Oscar Herrero for support during the data management phase (Clinical Research Associate, Novo Nordisk Pharma S.A.), and we extend our gratitude to the parents and children, without whom this study would not have been possible.
Footnotes
This trial has been registered at http://www.clinicaltrials.gov (identifier NCT00184769).
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.NAPRTCS: NAPRTCS 2008 Annual Report. Available at: http://spitfire.emmes.com/study/ped/index.htm Accessed June 3, 2009
- 2.Furth SL: Growth and nutrition in children with chronic kidney disease. Adv Chronic Kidney Dis 12: 366–371, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Kuizon BD, Salusky IB: Growth retardation in children with chronic renal failure. J Bone Miner Res 14: 1680–1690, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Karlberg J, Schaefer F, Hennicke M, Wingen AM, Rigden S, Mehls O: Early age-dependent growth impairment in chronic renal failure. European Study Group for Nutritional Treatment of Chronic Renal Failure in Childhood. Pediatr Nephrol 10: 283–287, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Rees L, Shaw V: Nutrition in children with CRF and on dialysis. Pediatr Nephrol 22: 1689–1702, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kari JA, Gonzalez C, Ledermann SE, Shaw V, Rees L: Outcome and growth of infants with severe chronic renal failure. Kidney Int 57: 1681–1687, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Parekh RS, Flynn JT, Smoyer WE, Milne JL, Kershaw DB, Bunchman TE, Sedman AB: Improved growth in young children with severe chronic renal insufficiency who use specified nutritional therapy. J Am Soc Nephrol 12: 2418–2426, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Abitbol CL, Zilleruelo G, Montane B, Strauss J: Growth of uremic infants on forced feeding regimens. Pediatr Nephrol 7: 173–177, 1993 [DOI] [PubMed] [Google Scholar]
- 9.Brewer ED: Growth of small children managed with chronic peritoneal dialysis and nasogastric tube feedings: 203-Month experience in 14 patients. Adv Perit Dial 6: 269–272, 1990 [PubMed] [Google Scholar]
- 10.Fine RN, Attie KM, Kuntze J, Brown DF, Kohaut EC: Recombinant human growth hormone in infants and young children with chronic renal insufficiency. Genentech Collaborative Study Group. Pediatr Nephrol 9: 451–457, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Schwartz GJ, Brion LP, Spitzer A: The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am 34: 571–590, 1987 [DOI] [PubMed] [Google Scholar]
- 12.Hernández M, Fundación Francisco Orbegozo: Growth charts [in Spanish], 3rd Ed., Madrid, Ergon, 2002 [Google Scholar]
- 13.Argente J, Barrios V, Chowen JA, Sinha MK, Considine RV: Leptin plasma levels in healthy Spanish children and adolescents, children with obesity, and adolescents with anorexia nervosa and bulimia nervosa. J Pediatr 131: 833–838, 1997 [DOI] [PubMed] [Google Scholar]
- 14.Argente J, Barrios V, Pozo J, Munoz MT, Hervas F, Stene M, Hernandez M: Normative data for insulin-like growth factors (IGFs), IGF-binding proteins, and growth hormone-binding protein in a healthy Spanish pediatric population: age- and sex-related changes. J Clin Endocrinol Metab 77: 1522–1528, 1993 [DOI] [PubMed] [Google Scholar]
- 15.Hernandez M, Sanchez E, Sobradillo B, Rincon JM, Narvaiza JL: A new method for assessment of skeletal maturity in the first 2 years of life. Pediatr Radiol 18: 484–489, 1988 [DOI] [PubMed] [Google Scholar]
- 16.Ledermann SE, Shaw V, Trompeter RS: Long-term enteral nutrition in infants and young children with chronic renal failure. Pediatr Nephrol 13: 870–875, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Fine RN, Ho M, Tejani A, Blethen S: Adverse events with rhGH treatment of patients with chronic renal insufficiency and end-stage renal disease. J Pediatr 142: 539–545, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Mahan JD, Warady BA: Assessment and treatment of short stature in pediatric patients with chronic kidney disease: A consensus statement. Pediatr Nephrol 21: 917–930, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Mencarelli F, Kiepe D, Leozappa G, Stringini G, Cappa M, Emma F: Growth hormone treatment started in the first year of life in infants with chronic renal failure. Pediatr Nephrol 24: 1039–1046, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Karlberg J: On the modelling of human growth. Stat Med 6: 185–192, 1987 [DOI] [PubMed] [Google Scholar]
- 21.Tonshoff B, Kiepe D, Ciarmatori S: Growth hormone/insulin-like growth factor system in children with chronic renal failure. Pediatr Nephrol 20: 279–289, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Argente J, Caballo N, Barrios V, Munoz MT, Pozo J, Chowen JA, Morande G, Hernandez M: Multiple endocrine abnormalities of the growth hormone and insulin-like growth factor axis in patients with anorexia nervosa: Effect of short- and long-term weight recuperation. J Clin Endocrinol Metab 82: 2084–2092, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Argente J, Caballo N, Barrios V, Pozo J, Munoz MT, Chowen JA, Hernandez M: Multiple endocrine abnormalities of the growth hormone and insulin-like growth factor axis in prepubertal children with exogenous obesity: Effect of short- and long-term weight reduction. J Clin Endocrinol Metab 82: 2076–2083, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Lindblad YT, Axelsson J, Barany P, Celsi G, Lindholm B, Qureshi AR, Carrea A, Canepa A: Hyperinsulinemia and insulin resistance, early cardiovascular risk factors in children with chronic kidney disease. Blood Purif 26: 518–525, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Mak RH, Haycock GB, Chantler C: Glucose intolerance in children with chronic renal failure. Kidney Int Suppl 15: S22–S26, 1983 [PubMed] [Google Scholar]
- 26.Besbas N, Ozaltin F, Coskun T, Ozalp S, Saatci U, Bakkaloglu A, El Nahas AM: Relationship of leptin and insulin-like growth factor I to nutritional status in hemodialyzed children. Pediatr Nephrol 18: 1255–1259, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Daschner M, Tonshoff B, Blum WF, Englaro P, Wingen AM, Schaefer F, Wuhl E, Rascher W, Mehls O: Inappropriate elevation of serum leptin levels in children with chronic renal failure. European Study Group for Nutritional Treatment of Chronic Renal Failure in Childhood. J Am Soc Nephrol 9: 1074–1079, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Arbeiter AK, Buscher R, Petersenn S, Hauffa BP, Mann K, Hoyer PF: Ghrelin and other appetite-regulating hormones in paediatric patients with chronic renal failure during dialysis and following kidney transplantation. Nephrol Dial Transplant 24: 643–646, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Szczepanska M, Szprynger K, Mazur B, Zwolinska D, Kilis-Pstrusinska K, Makulska I: Plasma ghrelin levels in children with chronic renal failure on peritoneal dialysis. Perit Dial Int 27: 61–66, 2007 [PubMed] [Google Scholar]
- 30.Nusken KD, Groschl M, Rauh M, Stohr W, Rascher W, Dotsch J: Effect of renal failure and dialysis on circulating ghrelin concentration in children. Nephrol Dial Transplant 19: 2156–2157, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Ozkaya O, Buyan N, Bideci A, Gonen S, Ortac E, Fidan K, Cinaz P, Soylemezoglu O: Osteoprotegerin and RANKL serum levels and their relationship with serum ghrelin in children with chronic renal failure and on dialysis. Nephron Clin Pract 105: c153–c158, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Yoshimoto A, Mori K, Sugawara A, Mukoyama M, Yahata K, Suganami T, Takaya K, Hosoda H, Kojima M, Kangawa K, Nakao K: Plasma ghrelin and desacyl ghrelin concentrations in renal failure. J Am Soc Nephrol 13: 2748–2752, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Asakawa A, Inui A, Fujimiya M, Sakamaki R, Shinfuku N, Ueta Y, Meguid MM, Kasuga M: Stomach regulates energy balance via acylated ghrelin and desacyl ghrelin. Gut 54: 18–24, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel L, Webb NJ, Bradbury MG, Zaman N, Smith P, Lewis MA, Postlethwaite RJ, Price DA, Clayton PE: Serum leptin and IGF-I during growth hormone treatment in chronic renal failure. Pediatr Nephrol 17: 643–647, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Mehls O, Ritz E, Hunziker EB, Eggli P, Heinrich U, Zapf J: Improvement of growth and food utilization by human recombinant growth hormone in uremia. Kidney Int 33: 45–52, 1988 [DOI] [PubMed] [Google Scholar]
- 36.Santos F, Chan JC, Hanna JD, Niimi K, Krieg RJ, Jr, Wellons MD: The effect of growth hormone on the growth failure of chronic renal failure. Pediatr Nephrol 6: 262–266, 1992 [DOI] [PubMed] [Google Scholar]
- 37.van der Sluis IM, Boot AM, Nauta J, Hop WC, de Jong MC, Lilien MR, Groothoff JW, van Wijk AE, Pols HA, Hokken-Koelega AC, de Muinck Keizer-Schrama SM: Bone density and body composition in chronic renal failure: Effects of growth hormone treatment. Pediatr Nephrol 15: 221–228, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Boot AM, Nauta J, de Jong MC, Groothoff JW, Lilien MR, van Wijk JA, Kist-van Holthe JE, Hokken-Koelega AC, Pols HA, de Muinck Keizer-Schrama SM: Bone mineral density, bone metabolism and body composition of children with chronic renal failure, with and without growth hormone treatment. Clin Endocrinol (Oxf) 49: 665–672, 1998 [DOI] [PubMed] [Google Scholar]