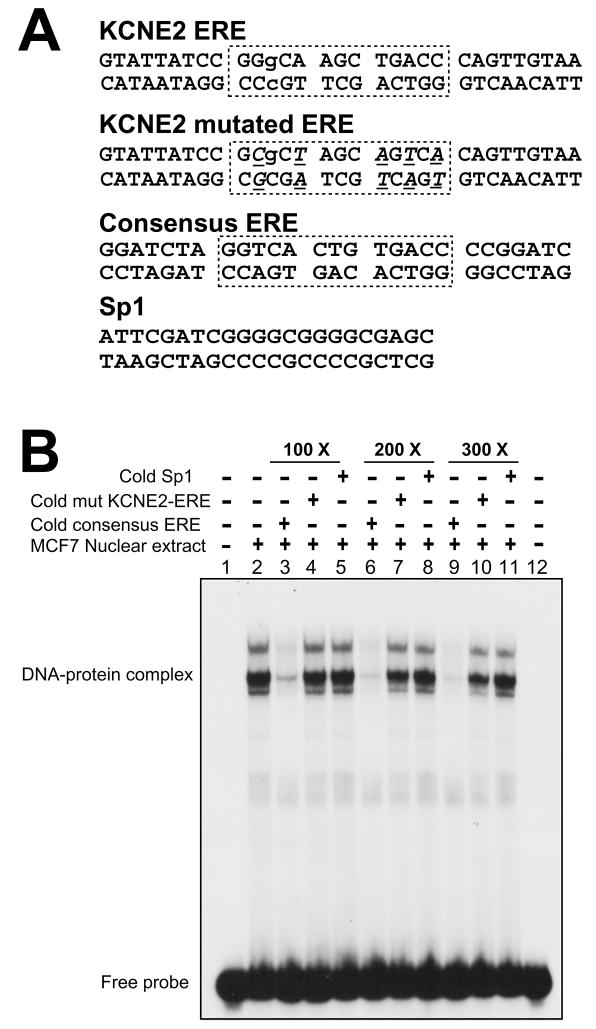
Figure 7.
Direct interaction of ERα with the ERE of the KCNE2 gene. (A), Duplex oligos used in EMSA. Boxes delineate the KCNE2 ERE in wild type, mutated and the perfect consensus ERE of the commercial oligo (Santa Cruz Biotechnology). In the KCNE2 mutated ERE, mutations are underlined in italics. Lowercase letters indicate the mismatch from the consensus ERE. (B), EMSA with nuclear extract of estrogen-treated MCF7 cells showing ERα binding with the KCNE2-ERE forming DNA-ERα complexes. Non-radioactive (cold) consensus ERE could compete with radiolabeled KCNE2-ERE for protein binding (lanes 3, 6 and 9). This competition is absent with mutated KCNE2-ERE (lanes 4, 7 and 10) and Sp1 duplex (lanes 5, 8 and 11). The dose dependence of the competition highlights the specificity of this reaction.