Table 2.
Stereospecific Suzuki Cross-coupling of Alkyl α-Cyanohydrin Triflates.a
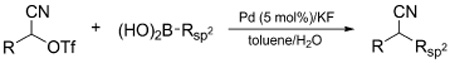 | ||||||
|---|---|---|---|---|---|---|
| entry | α-cyanohydrin | boronic acid | adduct | cat. (mol%) |
temp (°C) /time (h) |
yield (%)b |
| 1 | 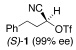 |
2 | 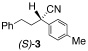 |
A | 40/20 | 91 (98% ee) |
| 2 | A | 40/24 | 93 (94% ee) |
|||
| 3 | 1 | B | 21/20 | 74 | ||
| 4 | 1 | A | 21/48 | 82 | ||
| 5 | 1 | A | 40/20 | 51c | ||
| 6 | 1 | A | 40/20 | 76 | ||
| 7 | 1 | B | 21/20 | 68 | ||
| 8 | 1 | A | 40/20 | 94 | ||
| 9 | 2 | B | 21/20 | 31 | ||
| 10 | 2 | B | 21/20 | 78 | ||
| 11 | 1 | A | 40/36 | 21 | ||
| 12 | 1 | 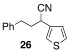 |
A | 40/20 | 56 | |
| 13 | 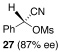 |
9 | 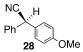 |
C | 40/24 | 84 (80% ee) |
Reaction conditions: triflate/mesylate (0.15 mmol), boronic acid (0.3 mmol), Pd catalyst (5 mol%), and KF (0.6 mmol) in toluene (2 mL)/H2O (10 µL).
Enantiomeric excess determined by chiral HPLC.
Using K3PO4·H2O (0.6 mmol) instead of KF; same conditions using KF (0.6 mmol) gave 33% yield.
