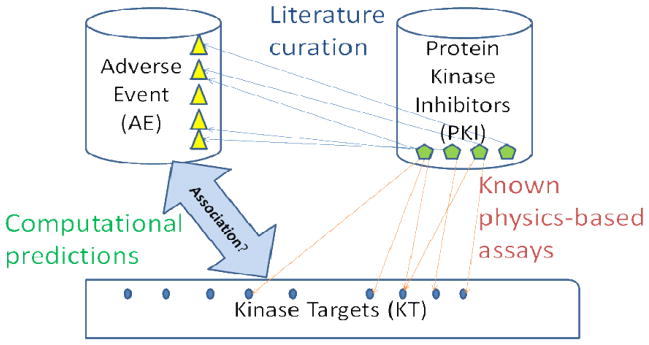
Figure 1. Illustration of raw data and their relationship in this study.
The frequencies of adverse events (AEs) associated with kinase inhibitors were curated from three sources (Thompson Physician Desk Reference, PharmGKB and PubMed). The dissociation constants (Kd) of kinases were summarized from physico-chemical assays[8]. The computational method in this study considers all of these data to predict the association between AEs and targets of kinase inhibitors.