Abstract
Rational
Psychostimulant injections in rats have been shown to alter future performance in natural reward conditioning. These effects may represent a persistent impact of drugs on neurocircuits that interface cognitive and motivational processes, which may be further altered in neuropsychiatric conditions that entail increased addiction vulnerability.
Objective
This study investigated whether a rat model of schizophrenia with cocaine addiction vulnerability shows altered natural reward conditioning with or without prior cocaine exposure.
Methods
Adult rats with SHAM or neonatal ventral hippocampal lesions were given cocaine (15 mg/kg per day for 5 days) or saline injections, followed 7 days later by natural reward-conditioned learning. Over ten daily sessions, water-restricted rats were assessed for durations of head entries into a magazine during random water presentations, a conditioning stimulus phase predictive of the water reward, and an “inappropriate” phase when conditioning stimuli were absent and reward presentation would be delayed.
Results
Over repeated sessions, lesioned and SHAM rats showed similar reductions in total magazine entry durations, with similar increases in the allocations of entry times during the water presentation. However, lesioned rats, especially those exposed to cocaine, demonstrated reduced allocations of magazine entry times during the conditioning stimulus phase, and increased allocations during the inappropriate phase.
Conclusions
Intact natural reward motivation accompanied by deficient learning of complex contingencies to guide efficient reward approach may represent a form of impulsivity as an addiction vulnerability trait marker in an animal model of schizophrenia.
Keywords: Schizophrenia, Dual diagnosis, Substance use disorders, Addiction, Impulsivity, Prefrontal cortex, Nucleus accumbens, Hippocampus, Conditioned learning
Introduction
Contemporary theories of addiction, supported by ample neuroanatomical, neurochemical and behavioral data, suggest that addictive drugs exert reinforcing actions via pharmacological activity in neurocircuits governing appetitive responses to natural rewards such as food and sexual opportunities (Di Chiara et al. 1993; Schultz 2000; Spanagel and Weiss 1999). Investigations of the capacity of repeated doses of addictive drugs to exert changes in neural systems of the nucleus accumbens (NAc), prefrontal cortex (PfC) or ventral tegmental area (VTA)-dopamine system have identified alterations in neurotransmitter efflux, neuronal protein and receptor expression, intracellular signaling, neural firing properties and neuronal morphology (Gerdeman et al. 2003; Kalivas et al. 2003; Nestler et al. 2001; Nicola et al. 1996; Robinson et al. 2001; Self and Nestler 1995). On the one hand, these changes may in part instantiate increased motivational responses to addictive drugs, as a core process underlying the development of addiction (Cami and Farre 2003; Robinson and Berridge 1993). On the other hand, these changes may represent a form of learning, albeit aberrant that not only involves neural systems governing natural reward, but can manifest behaviorally as alterations in future natural reward-related learning (Everitt et al. 2001; Kelley et al. 2003; Olausson et al. 2003). In this manner, aspects of natural reward-related learning, tested after sub-chronic addictive drug exposure could represent indirect signs of the addiction process underway, and/or in animals with known predisposition to addiction vulnerability, may serve as behavioral trait markers of increased addiction vulnerability.
In exploring these hypotheses, we studied rats with neonatal ventral hippocampal lesions (NVHL) in a natural reward learning protocol 1 week after exposure to a 5-day regimen of cocaine (COC) injections. NVHL rats have been studied by several groups as a comprehensive animal model of schizophrenia. NVHL rats show post-adolescent onset of neuroleptic-responsive, positive-like symptoms, such as hyper-responsivity of dopamine-mediated behaviors (Lipska et al. 1993; Lipska and Weinberger 1994) accompanied by a number of schizophrenia-related cognitive and negative-like symptoms (Becker et al. 1999; Chambers et al. 1996; Grecksch et al. 1999; Lipska et al. 1995). In applying this animal model toward understanding increased substance use disorder (SUD) comorbidity in schizophrenia, NVHL rats show increased vulnerability to developing an addicted phenotype in acquisition, maintenance and post-withdrawal phases of COC self-administration (Chambers and Self 2002). In the present study we tested rats with NVHL or SHAM lesions and recent history of sub-chronic COC exposure (15 mg/kg per day for 5 days) or saline (SAL) injections, on subsequent natural reward-conditioned learning. Using this protocol occurring over ten daily trials, water-restricted rats learned to put their heads into a magazine during the unconditioned stimulus (UCS) when water was available for 5 s, and during the conditioning stimulus (CS) when a compound visual-auditory cue was presented for 5 s immediately preceding the onset of the UCS. The CS–UCS sequence was initiated on a random schedule. Rats also learned not to approach the magazine at “inappropriate” times (INA interval), when neither the CS nor UCS were presented, and reward approach resulted in a temporal delay in the onset of the next CS–UCS sequence. Analysis of the total durations of magazine entries and the allocations from this total occurring during the UCS, CS, and INA phases of the paradigm were conducted to discern lesion and/or COC history effects on natural reward conditioning.
Materials and methods
Animals
Subjects were male Sprague–Dawley pups born in the laboratory from pregnant dams arriving in the laboratory at 13–17 days gestation. Litters and subsequent post-weaning groups were maintained under standard feeding conditions with lights on at 0700 hours for 12 h. On post-natal day (PD) 7, male pups weighing between 16 g and 19 g were removed from their mothers for 1–2 h for NVHL/SHAM surgeries, with balanced lesion assignments per litter. Post-lesioning, litters were left undisturbed until weaning on PD 21, when pups were housed in groups of four per cage. At PD 42–45, groups were again reduced to two per cage. All surgical procedures and behavioral protocols were approved by the Yale University School of Medicine Animal Care and Use Committee and in accordance with NIH guidelines in “Principles of Laboratory Animal Care” (National Institutes of Health publication number 85–23, rev. 1996).
Neonatal ventral hippocampal lesions
Pups were randomly assigned to receive either bilateral SHAM or ibotenic acid lesions of the ventral hippocampus, according to the protocol developed by Lipska et al. (1993). Briefly, pups were anesthetized by hypothermia on ice for 15–20 min and secured with tape onto a customized platform mounted in an adult rat stereotaxic frame (David Kopf Instruments, Tujunga, CA). Head position was maintained by the tape such that the dorsal surface was approximately horizontal. A longitudinal incision was made on the dorsal surface of the head, and a Hamilton syringe needle (26S gauge) was lowered through the skull into the ventral hippocampal formation (AP −3.0 mm, ML± 3.5 mm, and VD −5.0 mm relative to bregma). Rats assigned to the lesioned group received 3.0 μg ibotenic acid (Sigma, St Louis, MO) in 0.3 μl artificial cerebrospinal fluid (CSF) vehicle over 135 s, whereas SHAM rats received only artificial CSF, via a Harvard Apparatus microinfusion pump. The needle was left in place for 3 min after infusion to prevent needle track backflow. The surgical wound was closed using Ethicon 4.0 silk sutures, and the pups were monitored and warmed with a heating pad before being returned to their mothers.
Injections
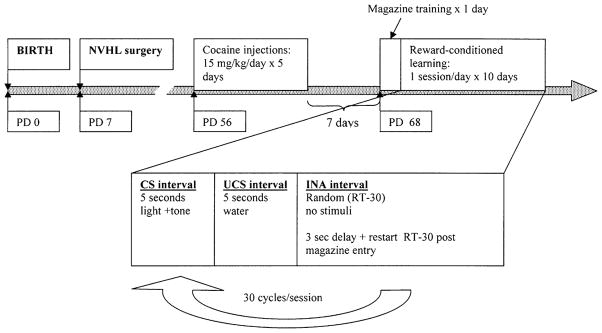
Cocaine hydrochloride (Sigma, St Louis, MO) was dissolved in 0.9% sterile SAL at a concentration of 15 mg/ml and injected as 1.0 ml/kg per rat i.p. This dose was chosen based on a prior study (Taylor and Jentsch 2001) showing alterations in natural reward-conditioned approach in healthy rats after COC 15 mg/kg per day for 5 days. As shown in Fig 1, beginning on PD 56, between 0900 hours and 1200 hours, COC or SAL injections were given once daily for five consecutive days in the animal housing room, with the last injection occurring 1 week prior to behavioral testing.
Fig. 1.
Experimental timeline according to rat age. PD post-natal day
Apparatus
Reward-conditioned learning was assessed in a room separate from the animal housing facility where animals received injections. Eight operant conditioning chambers (ENV-008CT, Med Associates, Inc., E. Fairfield, VT), measuring 30×20×25 cm3, with grid floors were equipped with a mechanical liquid dipper (0.06-ml cup size) presenting water into a recessed magazine on the right wall. A photocell in the ceiling of the recess measured duration of head entries. A 2.5-W, 24-V light located above the magazine and a Sonalert tone (10 kHz) generator emitting a 65-dB tone provided the compound visual and auditory CS. Operant conditioning chambers were housed in sound-attenuating cubicles measuring 61×62×41 cm3 equipped with a white-noise generator and ventilating fan to minimize external sound. A PC-compatible computer with Med Associates, Inc. (Georgia, VT) software controlled the chambers.
Reward-conditioned learning
Subjects were water restricted to only 30 min/day (afternoon) of access to water bottles in their home cages 5 days after the last COC/SAL injection. Beginning 7 days post-injections, animals underwent a single initial magazine training session for familiarization to the training apparatus and to facilitate awareness of intermittent water presentations in the recessed magazine. Under constant chamber house lighting, activation of the dipper presented rats with 5-s access to 0.06 ml water in 20-s intervals (FT-20) for a total of 100 UCS presentations. Total times for head entry into the magazine (1) during presentation of the UCS (UCS interval) and (2) between water presentations (non-UCS interval) were recorded.
After the initial magazine training session, rats underwent ten consecutive days (one session per day) of reward-conditioned learning. In these sessions, animals were presented with a 5-s CS interval (CS: house lights off, stimulus lights above magazine and auditory tone on) immediately followed by the UCS interval (house lights back on, stimulus light off, tone off, water dipper raised providing access to 0.06 ml water) for 5 s. The CS–UCS sequence was initiated under a random-time, 30-s (RT-30) schedule. Total head entry times per session for each of the 5-s CS-intervals and UCS-intervals were recorded along with the durations of head entries when neither the CS nor UCS were activated (INA interval). Head entry during the INA interval (termed “inappropriate” head entries), resulted in a 3-s delay in the onset of the RT-30 leading to the CS–UCS sequence, beginning only when the head moved out of the magazine. Repeated entries during the INA interval resulted in repeated delays in the CS–UCS presentation sequence and thus served as a form of mild punishment for indiscriminant magazine head entries. Healthy rats training on this schedule show a progressive increase in total time per session of magazine entry during the CS-interval and UCS-interval, and a decrease in head entry during the INA interval (Olmstead et al. 1998), demonstrating learning of the CS as a conditioned reinforcer (Robbins 1977).
Data analysis
For assessment of magazine entries during the initial magazine training session, two-way analyses of variance (ANOVAs; independent variables: lesion status and drug history) were performed on the total duration of magazine entry during water presentation (UCS interval) or otherwise (non-UCS interval) as separate analyses. Over the subsequent 10 days of reward-conditioned learning, three-way repeated-measures ANOVAs (independent variables: lesion status and drug history; within-subjects variable: days) were used to assess the following dependent variables in separate analyses: total time of magazine entry per session, UCS phase approach as a percentage of total time of magazine entry per session, CS phase approach as a percentage of total time of magazine entry per session, INA phase approach as a percentage of total time of magazine entry per session. Taken as percentage allocations from the total magazine entry durations, the UCS, CS, and INA phase measures allowed assessment of the relative specificity of targeting of head entries to each conditioning phase. Significant main effects of lesion or drug history were followed by post-hoc analysis using the Tukey procedure to specify between group differences. All data are expressed as means±SEMs, and significance was reported at P<0.05.
Results
Lesion verification
Forty-two neonatal rat pups received ibotenic acid lesions while 20 received SHAM operations. Half of the animals from each group were subsequently randomized to receive either COC or SAL injections. Of the 21 rats assigned to the NVHL–SAL group, 10 were found to have appropriate lesions, while 9 of 21 of the NVHL–COC group had appropriate lesions. None of the 20 SHAM rats showed evidence of excitotoxin-induced injury. Figure 2 summarizes the minimal versus maximal extent of the lesions across the 19 lesioned rats included in the study. Appropriate hippocampal damage was rated upon visualization of neuronal loss in hippocampal tissue in at least two consecutive sections spaced approximately 400 μm apart. The smallest lesions were confined to the area of confluence of the dorsal and ventral CA3 regions. The largest lesion impacted the tip of the rostral–dorsal CA3 region, and extended ventrally and caudally into the ventral CA3, the ventral CA1 and subiculum and ventral–caudal DG regions. In most sections, the lateral ventricles were enlarged. In some cases, minor gliosis was noted and allowed on the lateral aspect of thalamic capsule medial to the hippocampus. Of the NVHL–SAL- and NVHL–COC-assigned rats, 11 and 12, respectively, were excluded from analysis due to the presence of only unilateral lesions or damage extending into the cortex, anterior-dorsal hippocampus, amygdala, or lateral thalamus.
Fig. 2.
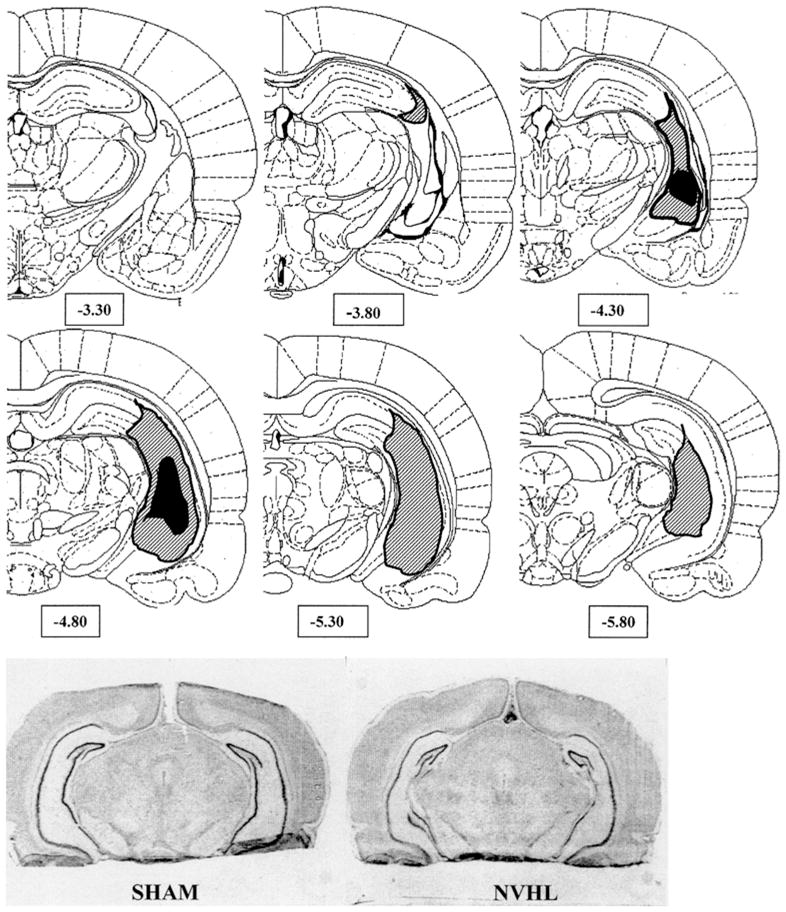
Hemi-coronal representations show the extremes of ibotenic-acid damage in rats with neonatal ventral hippocampal lesion (NVHL) included in the behavioral analysis. Striped areas represent the largest area of visually identified tissue change (on either side), while the black areas represent the smallest lesion extent. Numbers below sections indicate distance posterior to bregma based on coordinates of Paxinos and Watson. Photomicrographs at the bottom show representative NVHL versus SHAM brains. The NVHL brain shows hippocampal atrophy, structural distortion, and loss of neurons in hippocampal cell layers, particularly CA3 in the ventral medial regions
Initial magazine training
In their first experience in the training apparatus, SHAM–SAL (n=10), NVHL–SAL (n=10), SHAM–COC (n=10) and NVHL–COC (n=9) groups were assessed for total time of head entry into the magazine during the regular presentation of the UCS (water dipper raised for 5 s every 15 s × 100) and between UCS presentations (non-UCS interval). Durations of head entry during UCS presentations were 209.0±33s(SHAM–SAL),214.8±28(NVHL–SAL),266.8± 34.5 (SHAM–COC) and 185.4±36 (NVHL–COC). Durations of head entry during non-UCS intervals were 714.1± 104 s (SHAM–SAL), 694.7±67 (NVHL–SAL), 792.2±82 (SHAM–COC) and 617.8±81 (NVHL–COC). Two-way ANOVAs performed on the UCS and non-UCS interval data separately found no significant effects of lesion status, COC history or their interactions on these measures.
Reward-conditioned learning
As shown in Table 1, total durations of magazine entries per session were reduced similarly across all groups with repeated sessions (days: F9,315=44, P<0.001), as indicated by non-significant main effects of lesion status or drug exposure. A significant days×drug history interaction (F9,315= 2.3, P<0.05) was indicative of some differential effect of COC on these learning patterns, but without producing an overtly identifiable pattern difference attributable to any of the four rat groups. Examination of the UCS data indicates that these reductions in total magazine entry durations were accompanied by a general increase in the specificity of targeting of magazine entries to the time of water availability (Table 2). Upon repeated sessions, all rat groups showed similar increases in the percentage of total magazine entry time that occurred during the water (UCS) presentation (days: F9,315=154, P<0.001), where lesion status and drug history had no significant effects or interactions.
Table 1.
Total time of magazine entry (seconds/day). Total durations of magazine entries summed over unconditioned stimulus (UCS), conditioned stimulus (CS) and inappropriate (INA) phases per experimental session. Data are expressed as group means±SEM. All rat groups showed reductions in total magazine entry times across the 10 days of conditioned learning, reflecting similar evolving patterns of non-specific magazine exploration and/or water reward seeking
| Day | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| SHAM–SAL | 1388±235 | 893±129 | 473±50 | 442±36 | 386±24 | 348±17 | 343±15 | 347±15 | 350±18 | 372±19 |
| NVHL–SAL | 1194±237 | 856±211 | 535±84 | 418±26 | 368±29 | 380±30 | 335±19 | 320±29 | 333±27 | 283±22 |
| SHAM–COC | 1140±129 | 593±94 | 552±90 | 508±83 | 345±18 | 379±24 | 342±21 | 354±24 | 352±15 | 335±20 |
| NVHL–COC | 1041±144 | 697±157 | 670±109 | 690±118 | 447±52 | 398±31 | 377±39 | 369±22 | 382±26 | 367±31 |
Table 2.
Unconditioned stimulus (UCS) phase approach (percentage of total magazine entry time). Percentage of total magazine entry durations per session occurring during the UCS phase (water presentation). Data are expressed as group means±SEM of (UCS phase entry duration/total entry duration)×100 calculated for each rat per session. All groups showed similar increases in the allocations of magazine entries targeting the water presentation phase
| Day | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| SHAM–SAL | 7±1 | 16±2 | 33±3 | 35±3 | 39±2 | 43±2 | 43±2 | 42±2 | 43±2 | 40±2 |
| NVHL–SAL | 8±1 | 19±4 | 28±3 | 34±2 | 40±3 | 39±3 | 43±2 | 45±4 | 43±3 | 49±2 |
| SHAM–COC | 7±1 | 25±4 | 30±4 | 32±4 | 42±3 | 39±3 | 43±3 | 43±3 | 42±2 | 44±2 |
| NVHL–COC | 7±1 | 17±3 | 23±4 | 25±4 | 34±5 | 36±3 | 37±4 | 37±2 | 38±3 | 41±3 |
However, as shown in Fig. 3, NVHLs impaired learning associated with increases in the proportional duration of magazine entries occurring during the CS interval predictive of water presentation. Thus, while a general increase in the allocation of magazine entry durations during the CS interval was observed across all subjects (days: F9,315=175.8, P<0.001), the main effect of lesion (F1,35=21.3, P<0.001) and days×lesion status interaction (F9,315=4.7, P<0.001) were significant in association with a significant days×drug history interaction (F9,315=2.5, P<0.01). By post-hoc analysis, the NVHL–COC group showed significantly lower durations of magazine entries during the CS interval than either the SHAM–SAL or SHAM–COC groups (P<0.01), while none of the other groups was mutually different.
Fig. 3.
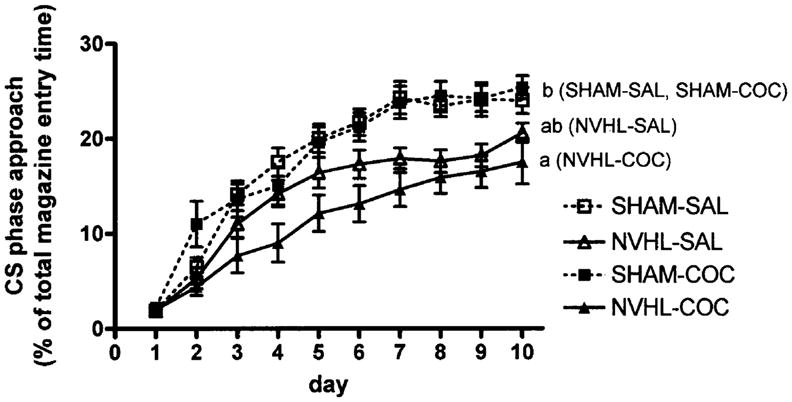
Percentage of total magazine entry durations per session occurring during the conditioned stimulus (CS) phase (5 s light and tone predictive of water presentation). Data are expressed as group means±SEM of (CS phase entry duration/total entry duration)×100, calculated for each rat per session. Neonatal ventral hippocampal lesions (NVHLs) reduced the CS-phase magazine-approach allocations (repeated-measures ANOVA, P<0.001), where NVHL–COC rats (a) differed from SHAM–SAL and SHAM–COC rats (b) at P<0.01 by post-hoc testing, and NVHL–SAL rats did not differ from the other groups (ab)
The capacity of all rat groups to suppress INA magazine entry during conditioned learning is shown in Fig. 4 (days: F9,315=189.7, P<0.001). Here, NVHLs were associated with an increased proportion of total magazine entry times occurring during the INA interval (lesion: F1,35=6.4, P<0.05) in association with a significant day×drug history interaction (F9,315=2.0, P<0.05). Post-hoc analysis revealed significant differences between NVHL–COC and SHAM–COC groups (P<0.05).
Fig. 4.
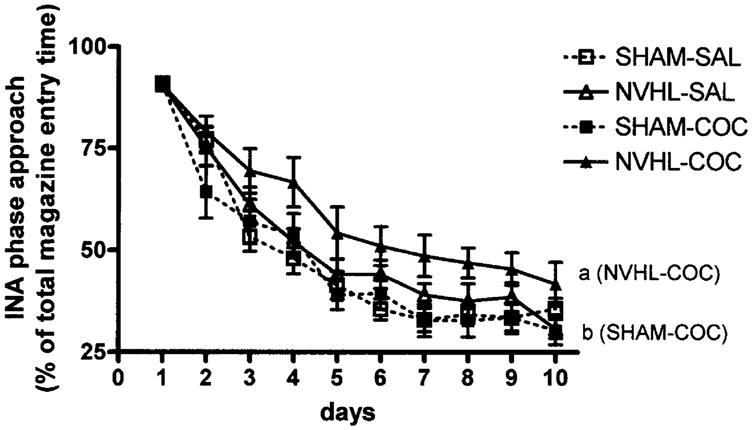
Percentage of total magazine entry durations per session occurring during the inappropriate (INA) phase [no stimuli, magazine entries produce delay in conditioned stimulus (CS)–unconditioned stimulus (UCS) sequence]. Data are expressed as group means±SEM of (INA phase entry duration/total entry duration)×100 calculated for each rat per session. Neonatal ventral hippocampal lesions (NVHLs) increased the INA phase magazine approach allocations (repeated-measures ANOVA, P<0.05), where NVHL-COC rats (a) differed from SHAM-COC rats (b) at P<0.05 by post-hoc testing
Discussion
This study demonstrates that NVHLs impair the acquisition of reward-approach behavior during a randomly presented stimulus CS phase predictive of natural reward presentation, in association with heightened allocations of reward-approach behavior to the “inappropriate” INA phases of the protocol. These effects were embedded within a general learning phenomenon observed across all groups. While the total durations of magazine entries—summed over the CS, UCS and INA phases in each session—showed substantial declines with repeated sessions (Table 1), the proportion of total magazine entry durations occurring during the water presentation (UCS phase) increased similarly across all groups from below 8% in the first session to above 40% by session 10 (Table 2). Thus, although both SHAM and NVHL rats showed similar learning capacities for increasing the specificity of reward-approach behavior to the water presentation (UCS phase), this behavior was less effectively shaped by the learning of anticipatory magazine entries during the CS phase (Fig. 3) in association with increased proportions of indiscriminate magazine entry durations in NVHL rats (Fig. 4).
An important consideration is whether NVHL-induced alterations in locomotor behavior or natural reward motivation could have contributed to these results. Among the initial findings characterizing the NVHL syndrome was hyperlocomotor responses to mild stressors and contextual novelty (Lipska et al. 1993), while subsequent studies identified decreased preference for a sweetened water supply during water restriction (Le Pen et al. 2002), but increased rates of instrumental responding for sucrose reward during food restriction (Chambers and Self 2002). Because lesion status did not produce significant effects on durations of UCS phase or non-UCS phase magazine entries in the initial magazine training session, or on total durations of magazine entries across the 10 days of conditioned learning, group differences in non-specific locomotor activity, if present, did not translate into differences in exploration of the magazine. Similarly, to the extent that total durations of magazine entries over conditioning may reflect motivated effort or non-specific seeking behavior for the water reward, the absence of lesion effects on this measure, or on the proportional durations of magazine entries during the water presentation (UCS phase), do not support an interpretation whereby lesion effects on the CS or INA measures resulted from significant differences in reward motivation per se. However, since NVHL rats show profound deficits in working memory performance measured by food reward seeking on a radial arm maze (Chambers et al. 1996), but increased lever pressing for food reward on a fixed-ratio 3 schedule (Chambers and Self 2002), the present results may reflect abnormal functioning of neural systems that integrate cognitive with motivational processes. NVHLs may compromise efficient reward acquisition requiring more complex learned associations (e.g., CS–UCS associations), while increasing motivational responding under more simple contingencies (e.g., achieving increasing proportions of UCS-targeted approaches via greater proportions of indiscriminate INA phase approaches).
As an animal model of increased SUD vulnerability in schizophrenia, NVHL rats self-administering COC show increased drug-paired lever presses in acquisition, incidence of binging-like patterns of drug intake, increased drug seeking after drug withdrawal, and COC-induced reinstatement of drug seeking after extinction of drug seeking (Chambers and Self 2002). The lesion-induced alterations in cognitive/motivational performance identified in the present study may represent an endophenotypic-like trait marker of this addiction vulnerability. Since INA phase magazine entry results in a mild form of punishment in terms of a temporal delay in the onset of the CS–UCS sequence, NVHL effects on increasing the proportions of INA phase approaches, while reducing proportions of CS phase approaches, may model aspects of clinical constructs of impulsivity. Numerous studies have linked behavioral trait markers of impulsivity with SUDS in primary SUD patient populations (Bickel et al. 1999; McGrue et al. 2001; Petry 2001), psychiatric populations with SUD comorbidity (Anthony and Helzer 1991; Dervaux et al. 2001; Moeller et al. 2001) and periadolescent age groups with developmental-age vulnerability to addictions (Chambers et al. 2003; Ernst etal. 2003). Moreover, 3,4-methylendioxymeth-amphetamine (MDMA) may be associated with increased impulsivity in human users (Morgan 1998), and this drug produces increased INA-phase responding in rats studied in similar reward-conditioned protocols (Taylor and Jentsch 2001).
While depletion or reduction of serotonin system activity could represent a primary mechanism for MDMA-related effects on measures of impulsivity (Morgan 1998), or in some clinical populations thought to have intrinsic serotonin dysfunction (Krakowski 2003), increased impulsivity as a parameter of decision making or cognitive control has more generally been associated with PfC dysfunction across varieties of neuropsychiatric disorders including schizophrenia, bipolar disorder, traumatic brain injury, anti-social personality and others (Cummings 1995; Moeller et al. 2001). Accumulating data on the behavioral and neurobiological effects of NVHLs indicate that the adult syndrome entails changes in medial PfC regions that normally receive direct ventral hippocampal projections (Bernstein et al. 1999; Carr and Sesack 1996). For example, N-acetylaspartate concentrations, taken as markers of neuronal integrity (Bertolino et al. 2002) and stress-induced expression of brain derived neurotrophic factor (BDNF) mRNA expression, as a mediator of synaptic plasticity, are reduced in the medial PfC of NVHL rats (Molteni et al. 2001). Both major excitatory and inhibitory transmitter systems in the PfC also appear to be altered by NVHLs, as evidenced by alterations in splice variants of AMPA glutamate receptor mRNA (Stine et al. 2001), increased glutamate binding (Schroeder et al. 1999), decreased concentrations of the γ-aminobutyric acid (GABA) production enzyme GAD-67 mRNA (Lipska et al. 2003) and decreased pyramidal cell dendritic length and spine density (Lipska et al. 2001). Correlated with these molecular and cellular alterations are neurophysiological changes: NVHL rats show abnormal patterns and elevations of neuronal firing in medial PfC pyramidal neurons produced by VTA stimulation (O’Donnell et al. 2002) that is mirrored by similar abnormalities in firing in medium spiny neurons in the NAc (Goto and O’Donnell 2002). Notably, a medial PfC uncorrupted by direct lesioning in NVHL rats is required for both the expression of hyperlocomotive responses to novelty and psychostimulants (Lipska et al. 1998), and the VTA-stimulation-induced increases in NAc neuronal firing (Goto and O’Donnell 2004). Taken together, these studies point to the involvement of both gross developmental injury in the ventral hippocampus and more subtle secondary changes in PfC regions that project directly into reward-responsive subcortical circuits including the NAc and VTA, in the impulsive style of natural reward approach observed in the current study. However, secondary NVHL effects localized to the NAc and amygdala may also be involved since (a) intact ventral hippocampal afferents to the NAc gate both PfC and amygdalar excitatory input activity to the NAc (O’Donnell and Grace 1995); (b) the ventral hippocampus innervates amygdalar regions (e.g., basolateral nucleus) that in turn project to the NAc (Pitkanen et al. 2000; Wright et al. 1996) and (c) amygdalar function has been associated with reward-conditioned learning (Everitt et al. 1999; Hitchcott et al. 1997). Thus, primary neonatal ventral hippocampal injury may conspire with secondary developmental alterations distributed throughout frontal-cortical and temporal limbic circuits to create an addiction-prone and impulsive phenotype (Chambers et al. 2001; Jentsch and Taylor 1999).
The precise mechanism(s) by which impulsivity is associated with increased addiction vulnerability, whether on the level of biophysical neural networks or in the processing of motivational representations within frontal-subcortical systems remains unelucidated. A fundamental phenomenological question concerns whether or not increased impulsivity is an acquired trait that emerges in parallel to the process of addiction, or whether a pre-drug exposed subject with high impulsivity is at increased risk of an accelerated addiction process. In this study, the latter possibility is most strongly supported since the abnormal allocations of reward approach during both the CS and INA phases were identified as significant main effects of lesion status but not main effects of COC history or lesion×drug history interactions. However, in both of these analyses, and as visually apparent in Figs. 3, 4, post-hoc testing revealed that the NVHL group with COC history carried these effects most robustly, suggestive of some degree of cooperative or permissive influence of NVHLs and COC history in producing an impulsive-like pattern in reward-conditioned learning. Notably, with respect to the SHAM animals, this study did not replicate previous findings where prior doses of psychostimulants have been shown to facilitate CS-phase reward approach in non-lesioned rats (Harmer and Phillips 1998; Taylor and Jentsch 2001). Although significant day× drug interactions were identified in both the CS and INA analyses, these effects likely emerged in relation to the blunted CS and INA learning curves most evident in NVHL rats with COC history. Methodological differences may account for lack of COC effects in the SHAM animals, including possible uncharacterized effects of the SHAM lesioning procedure, differences in testing schedules, and the fact that in this study none of the rats were excluded from the conditioned learning protocol based on predetermined thresholds of performance in the magazine training session. Incorporation of an additional measure for determining group differences in the pharmacological efficacy of repeated COC injections, such as differences in locomotor sensitization, would have been useful in confirming the extent of COC activity in the SHAM rats. Nevertheless, under the conditions of this study, COC history tended to have greater effects in separating out the group performances of NVHL relative to those of SHAM rats, suggesting that NVH lesions could induce a baseline trait of indiscriminate reward approach in natural reward-conditioned learning that may be exacerbated by a history of repeated COC exposure. Analogous findings have been observed in terms of the locomotor activating effects of COC injections in a separate study (Chambers and Taylor 2004): NVHL rats not only show hyperlocomotive responses to single psychostimulant injections, but also show elevated sensitization curves with daily COC injections when compared with SHAMs. Thus, in both natural reward-conditioned learning and locomotor sensitization studies, repeated COC injections in NVHL rats may more potently exacerbate phenotypic expression of behavioral markers of addictive drug exposure, consistent with the more robust installment of an addicted phenotype in COC self-administration (Chambers and Self 2002).
In summary, this study showed that in natural reward-conditioned learning, NVHL rats show an intact capacity for refining reward approach behavior to the immediate presentation of a natural reward, but show reduced allocations of reward approach behavior guided by compound conditioning stimuli with greater proportions of indiscriminant approaches punished by temporal delays in reward delivery. These results may reflect a cognitive-motivational style resulting from abnormal PfC–NAc interactions secondary to the NVH lesion that operates most effectively under simple reward contingencies in association with impulsive-like reward approach patterns. Further behavioral and neurobiological investigations of NVHL rats and other animal models of mental illness in drug and motivational paradigms are needed for a better understanding of the causal mechanisms relating mental illness, impulsivity and increased addiction vulnerability.
Acknowledgments
This work was made possible by funding provided by NARSAD Young Investigator Award, NIDA PSTP (DA00167), Raymond E. Houk Scholarship (Indiana University) (R.A.C.), and NIDA (DA11717) (J.R.T.).
Contributor Information
R. Andrew Chambers, Email: robchamb@iupui.edu, Laboratory for Translational Neuroscience of Dual Diagnosis Disorders, Institute of Psychiatric Research, Indiana University School of Medicine, 791 Union Drive, Indianapolis, IN, 46202, USA, Tel.: +1-317-2781716, Fax: +1-317-2741365.
Rachel M. Jones, Division of Molecular Psychiatry, New Haven, CT, USA
Scott Brown, Department of Cognitive Science, UC Irvine, Irvine, CA, USA.
Jane R. Taylor, Division of Molecular Psychiatry, Connecticut Mental Health Center, Yale University School of Medicine, New Haven, CT, USA
References
- Anthony J, Helzer J. Syndromes of drug abuse and dependence. In: Robins L, Regier D, editors. Psychiatric disorders in America. Free; New York: 1991. pp. 116–154. [Google Scholar]
- Becker A, Grecksch G, Bernstein HG, Hoellt V, Bogerts B. Social behavior in rats lesioned with ibotenic acid in the hippocampus: quantitative and qualitative analysis. Psychopharmacology. 1999;144:333–338. doi: 10.1007/s002130051015. [DOI] [PubMed] [Google Scholar]
- Bernstein HG, Grecksch G, Becker A, Hoellt V, Bogerts B. Cellular changes in rats brain areas associated with neonatal hippocampal damage. Neuroreport. 1999;10:2307–2311. doi: 10.1097/00001756-199908020-00016. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Roffman JL, Lipska BK, van Gelderen P, Olson A, Weinberger DR. Reduced N-acetylaspartate in prefrontal cortex of adult rats with neonatal hippocampal damage. Cereb Cortex. 2002;12:983–990. doi: 10.1093/cercor/12.9.983. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: delay discounting in current, never and ex-smokers. Psychopharmacologia. 1999;146:447–454. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- Cami J, Farre M. Drug addiction. N Engl J Med. 2003;349:975–986. doi: 10.1056/NEJMra023160. [DOI] [PubMed] [Google Scholar]
- Carr DB, Sesack S. Hippocampal afferents to the rat prefrontal cortex: synaptic targets and relation to dopamine terminals. J Comp Neurol. 1996;369:1–15. doi: 10.1002/(SICI)1096-9861(19960520)369:1<1::AID-CNE1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Self DW. Motivational responses to natural and drug rewards in rats with neonatal ventral hippocampal lesions: an animal model of dual diagnosis schizophrenia. Neuropsychopharmacology. 2002;27:889–905. doi: 10.1016/S0893-133X(02)00365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR. Animal modeling dual diagnosis schizophrenia: sensitization to cocaine in rats with neonatal ventral hippocampal lesions. Biol Psychiatry. 2004;56:308–316. doi: 10.1016/j.biopsych.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Moore J, McEvoy JP, Levin ED. Cognitive effects of neonatal hippocampal lesions in a rat model of schizophrenia. Neuropsychopharmacology. 1996;15:587–594. doi: 10.1016/S0893-133X(96)00132-7. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Krystal JK, Self DW. A neurobiological basis for substance abuse comorbidity in schizophrenia. Biol Psychiatry. 2001;50:71–83. doi: 10.1016/s0006-3223(01)01134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J. Anatomic and behavioral aspects of frontal-subcortical circuits. Ann NY Acad Sci. 1995;769:1–13. doi: 10.1111/j.1749-6632.1995.tb38127.x. [DOI] [PubMed] [Google Scholar]
- Dervaux A, Bayle F, Laqueille X, et al. Is substance abuse in schizophrenia related to impulsivity, sensation seeking or anhedonia. Am J Psychiatry. 2001;158:492–494. doi: 10.1176/appi.ajp.158.3.492. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Acquas E, Tanda G, Cadoni C. Drugs of abuse: biochemical surrogates of specific aspects of natural reward. Biochem Soc Symp. 1993;59:65–81. [PubMed] [Google Scholar]
- Ernst M, Grant SJ, London ED, Contoreggi CS, Kimes AS, Spurgeon L. Decision making in adolescents with behavior disorders and adults with substance abuse. Am J Psychiatry. 2003;160:33–40. doi: 10.1176/appi.ajp.160.1.33. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Parkinson JA, Olmstead MC, Arroyo M, Robledo P, Robbins TW. Associative processes in addiction and reward: the role of amygdala-ventral striatal subsystems. Ann NY Acad Sci. 1999;877:412–438. doi: 10.1111/j.1749-6632.1999.tb09280.x. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behavior. Brain Res Rev. 2001;36:129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- Gerdeman GL, Partridge JG, Lupica CR, Lovinger DM. It could be habit forming: drugs of abuse and striatal synaptic plasticity. Trend Neurosci. 2003;26:184–192. doi: 10.1016/S0166-2236(03)00065-1. [DOI] [PubMed] [Google Scholar]
- Goto Y, O’Donnell P. Delayed mesolimbic system alteration in a developmental animal model of schizophrenia. J Neurosci. 2002;22:9070–9077. doi: 10.1523/JNEUROSCI.22-20-09070.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, O’Donnell P. Prefrontal lesion reverses abnormal mesoaccumbens response in an animal model of schizophrenia. Biol Psychiatry. 2004;55:172–176. doi: 10.1016/s0006-3223(03)00783-2. [DOI] [PubMed] [Google Scholar]
- Grecksch G, Bernstein HG, Becker A, Hoellt V, Bogerts B. Disruption of latent inhibition in rats with postnatal hippocampal lesions. Neuropsychopharmacology. 1999;20:525–532. doi: 10.1016/S0893-133X(98)00081-5. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Phillips GD. Enhanced appetitive conditioning following repeated pre-treatment with D-amphetamine. Behav Pharmacol. 1998;9:299–308. [PubMed] [Google Scholar]
- Hitchcott PK, Harmer CJ, Phillips GD. Enhanced acquisition of discriminative approach following intra-amygdala D-amphetamine. Psychopharmacology. 1997;132:237–246. doi: 10.1007/s002130050341. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontos-triatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K, Bowers S, Szumlinski K, Xi ZX, Baker D. Glutamate transmission and addiction to cocaine. Ann NY Acad Sci. 2003;1003:169–175. doi: 10.1196/annals.1300.009. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Andrzejewski ME, Baldwin AE, Hernanadez P, Pratt WE. Glutamate-mediated plasticity in corticostriatal networks: role in adaptive motor learning. Ann NY Acad Sci. 2003;1003:159–168. doi: 10.1196/annals.1300.061. [DOI] [PubMed] [Google Scholar]
- Krakowski M. Violence and serotonin: influence of impulse control, affect regulation, and social functioning. J Neuropsychiatry Clin Neurosci. 2003;15:294–305. doi: 10.1176/jnp.15.3.294. [DOI] [PubMed] [Google Scholar]
- Le Pen G, Gaudet L, Mortas P, Mary R, Moreau J. Deficits in reward sensitivity in a neurodevelopmental rat model of schizophrenia. Psychopharmacology. 2002;161:434–441. doi: 10.1007/s00213-002-1092-4. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Weinberger DR. Subchronic treatment with haloperidol and clozapine in rats with neonatal excitotoxic hippocampal damage. Neuropsychopharmacology. 1994;10:199–205. doi: 10.1038/npp.1994.22. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Jaskiw GE, Weinberger DR. Postpubertal emergence of hyperresponsiveness to stress and to amphetamine after neonatal excitotoxic hippocampal damage: a potential animal model of schizophrenia. Neuropsychopharmacology. 1993;9:67–75. doi: 10.1038/npp.1993.44. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Swerdlow NR, Geyer MA, Jaskiw GE, Braff DL, Weinberger DR. Neonatal excitotoxic hippocampal damage in rats causes post-pubertal changes in prepulse inhibition of startle and its disruption by apomorphine. Psychopharmacology. 1995;122:35–43. doi: 10.1007/BF02246439. [DOI] [PubMed] [Google Scholar]
- Lipska BK, al-Amin HA, Weinberger DR. Excitotoxic lesions of the rat medial prefrontal cortex. Effects on abnormal behaviors associated with neonatal hippocampal damage. Neuropsychopharmacology. 1998;19:451–464. doi: 10.1016/S0893-133X(98)00045-1. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Kolb B, Halim N, Weinberger DR. Synaptic abnormalities in prefrontal cortex and nucleus accumbens of adult rats with neonatal hippocampal damage. Schizophr Res. 2001;49:47. [Google Scholar]
- Lipska BK, Lerman DN, Khaing ZZ, Weinberger DR. The neonatal ventral hippocampal lesion model of schizophrenia: effects on dopamine and GABA mRNA markers in the rat midbrain. Eur J Neurosci. 2003;18:3097–3104. doi: 10.1111/j.1460-9568.2003.03047.x. [DOI] [PubMed] [Google Scholar]
- McGrue M, Iacono WG, Legrand LN, Malone S, Elkins I. Origins and consequences of age at first drink. Associations with substance-use disorders, disinhibitory behavior and psychopathology, and P3 amplitude. Alcohol Clin Exp Res. 2001;25:1156–1165. [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. Psychiatric aspects of impulsivity. Am J Psychiatry. 2001;158:1783–1793. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- Molteni R, Lipska B, Weinberger DR, Racagni G, Riva MA. Developmental and stress-related changes of neurotrophic factor gene expression in an animal model of schizophrenia. Mol Psychiatry. 2001;6:285–292. doi: 10.1038/sj.mp.4000865. [DOI] [PubMed] [Google Scholar]
- Morgan MJ. Recreational use of “ecstacy” (MDMA) is associated with elevated impulsivity. Neuropsychopharmacology. 1998;19:252–264. doi: 10.1016/S0893-133X(98)00012-8. [DOI] [PubMed] [Google Scholar]
- Nestler E, Barrot M, Self D. ΔFosB: a sustained molecular switch for addiction. Proc Natl Acad Sci U S A. 2001;98:11042–11046. doi: 10.1073/pnas.191352698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM, Kombian SB, Malenka RC. Psychostimulants depress excitatory transmission in the nucleus accumbens via presynaptic D1-like dopamine receptors. J Neurosci. 1996;16:1591–1604. doi: 10.1523/JNEUROSCI.16-05-01591.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell P, Grace AA. Synaptic interactions among excitatory afferents to the nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci. 1995;15:3622–3639. doi: 10.1523/JNEUROSCI.15-05-03622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell P, Lewis BL, Weinberger DR, Lipska B. Neonatal hippocampal damage alters electrophysiological properties of prefrontal cortical neurons in adult rats. Cereb Cortex. 2002;12:975–982. doi: 10.1093/cercor/12.9.975. [DOI] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Taylor JR. Repeated nicotine exposure enhances reward-related learning in the rat. Neuropsychopharmacology. 2003;28:1264–1271. doi: 10.1038/sj.npp.1300173. [DOI] [PubMed] [Google Scholar]
- Olmstead MC, Robbins TW, Everitt BJ. Basal forebrain cholinergic lesions enhance conditioned approach responses to stimuli predictive of food. Behav Neurosci. 1998;112:611–629. doi: 10.1037//0735-7044.112.3.611. [DOI] [PubMed] [Google Scholar]
- Petry NM. Substance abuse, pathological gambling, and impulsiveness. Drug Alcohol Depend. 2001;63:29–38. doi: 10.1016/s0376-8716(00)00188-5. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. Ann NY Acad Sci. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Relationship between reward-enhancing and stereotypical effects of psychomotor stimulant drugs. Nature. 1977;264:57–59. doi: 10.1038/264057a0. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Gorny G, Mitton E, Kolb B. Cocaine self-administration alters the morphology of dendrites and dendritic spines in the nucleus accumbens and neocortex. Synapse. 2001;39:257–266. doi: 10.1002/1098-2396(20010301)39:3<257::AID-SYN1007>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Schroeder H, Grecksch G, Becker A, Bogerts B, Hoellt V. Alterations of the dopaminergic and glutamatergic neurotransmission in adult rats with postnatal ibotenic acid hippocampal lesion. Psychopharmacology. 1999;145:61–66. doi: 10.1007/s002130051032. [DOI] [PubMed] [Google Scholar]
- Schultz W. Multiple reward signals in the brain. Nat Rev Neurosci. 2000;1:199–207. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- Self DW, Nestler EJ. Molecular mechanisms of drug reinforcement and addiction. Ann Rev Neurosci. 1995;18:463–495. doi: 10.1146/annurev.ne.18.030195.002335. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Weiss F. The dopamine hypothesis of reward: past and current status. Trend Neurosci. 1999;22:521–527. doi: 10.1016/s0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]
- Stine CD, Lu W, Wolf ME. Expression of AMPA receptor flip and flop mRNAs in the nucleus accumbens and prefrontal cortex after neonatal ventral hippocampal lesions. Neuropsychopharmacology. 2001;24:253–266. doi: 10.1016/S0893-133X(00)00212-8. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Jentsch JD. Repeated intermittent administration of psychomotor stimulant drugs alters the acquisition of Pavlovian approach behavior in rats: differential effects of cocaine, D-Amphetamine and 3,4-methylenedioxymethamphetamine(“Ecstasy”) BiolPsychiatry. 2001;50:137–143. doi: 10.1016/s0006-3223(01)01106-4. [DOI] [PubMed] [Google Scholar]
- Wright CI, Beijer AVJ, Groenewegen HJ. Basal amygdaloid complex afferents to the rat nucleus accumbens are compartmentally organized. J Neurosci. 1996;16:1877–1893. doi: 10.1523/JNEUROSCI.16-05-01877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]