Figure 5. IL-12 and IFNα promote histone hyperacetylation at the grzB and Eomes gene loci during CD8 T cell differentiation.
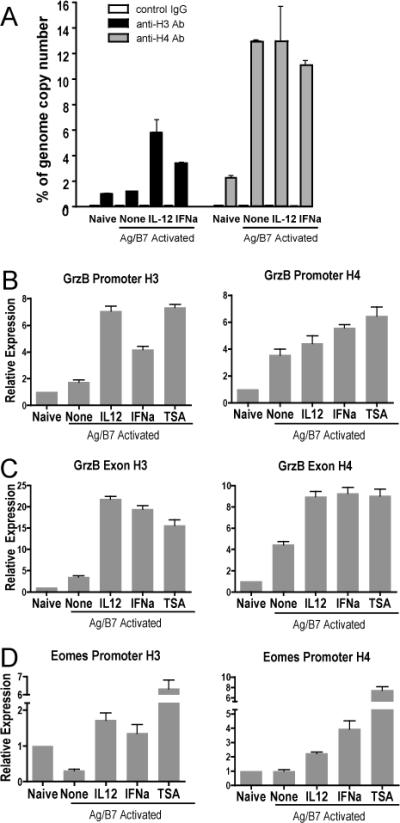
Purified CD44lo OT-I CD8 T cells were used as naïve control or were stimulated with Ag-B7 aAPC alone or with IL-12, IFNα or TSA (7.5ng/ml). Cells were harvested at 48h, processed, and examined by crosslinked-CHIP and qRT-PCR for acetylated histones H3 and H4. (A) Association of H3 and H4 histones with the grzB promoter, expressed as template copy numbers as a percent of whole genome copy number. Error bars show ranges for duplicate samples. (B-D) Association of H3 and H4 histones with the grzB promoter (B), grzB distal region (B) and the Eomes promoter (C) regions. Template copy numbers for each condition were internally normalized with their respective input control. Relative expression is calculated as the ratio of template copy numbers of each sample relative to the naïve control in each experiment, and is shown as mean with SEM of 2 or 3 independent experiments with duplicate samples for each. In comparison to naïve cells, activation with Ag-B7 resulted in significant increases of acetylated H3 and H4 histones with the promoter and exon regions of GrzB (p < 0.05), a significant decrease in acetylated H3 histone association with the Eomes promoter (p < 0.001), and no change in association of acetylated H4 with the Eomes promoter (p = 0.5). In comparison to cells stimulated with just Ag-B7, association of acetylated H3 and H4 histones was significantly greater when IL-12, IFNα or TSA was added (p < 0.03), with one exception. The exception was acetylated H4 association with the GrzB promoter, where the additions did not cause a significant increase (p > 0.05).
