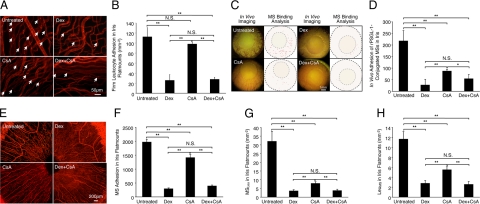
Figure 7.
In vivo and ex vivo molecular evaluation of anti-inflammatory treatment success. Representative micrographs depicting iridal flat mounts of uveitic animals that were topically treated with eye drops of Dex or CsA. rPSGL-1-conjugated MSs were injected through the tail vein and 30 minutes later animals were perfused with rhodamine ConA to stain the vasculature and adherent leukocytes. A: Ex vivo visualization of firmly adhering leukocytes in the iridal microvasculature with or without Dex treatment. Arrows indicate firmly adhering leukocytes (bright red spots) inside the iridal microvessels. B: Quantitative comparison of the numbers of accumulated leukocytes in the iridal microvasculature per surface area (mm−2), with or without topical anti-inflammatory treatments. **P < 0.01; N.S., not significant. C: Video-micrographs of rPSGL-1-conjugated MS accumulation in vivo of uveitic animals with or without topical treatment, 30 minutes after MS injection. In the video still images (left), yellow-green spots delineate adhering MSs. In the ImageJ pictures (right), each dot indicates automated counts of adhering MSs in the iris vessels of normal and iritic animals. D: Quantitative comparison of in vivo MS accumulation in the total iridal vasculature of iritis animals using intravital microscopy, with or without topical anti-inflammatory treatments. *P < 0.05; **P < 0.01; N.S., not significant. E: Ex vivo visualization of accumulated MSs (yellow-green spots) in the iris flat mounts, with or without anti-inflammatory treatment. F: Quantitative comparison of ex vivo MS accumulation in total iridal flat mounts of iritis animals, with or without topical anti-inflammatory treatment. **P < 0.01; N.S., not significant. G: The number of MSs binding to leukocytes (MSLeu) in iridal microvasculature per surface area (mm−2), with or without topical anti-inflammatory treatments. **P < 0.01; N.S., not significant. H: The number of leukocytes binding to MSs (LeuMS) in iridal microvasculature per surface area (mm−2), with or without topical anti-inflammatory treatment. **P < 0.01; N.S., not significant.