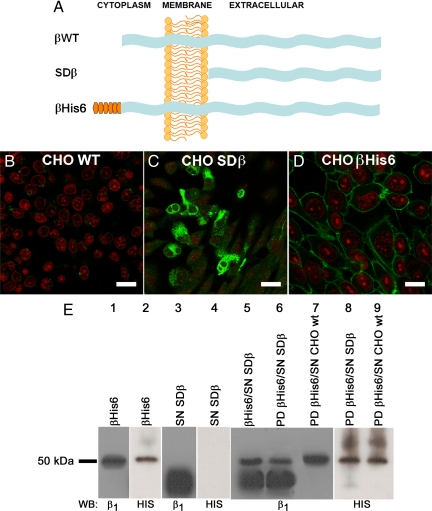
Figure 2.
β Subunits interact in vitro. (A) Scheme of the wild-type and recombinant β subunits used in this study. Shown are the wild-type β subunit (βWT, top), the soluble β subunit consisting only of the extracellular domain (SDβ, middle), and the β subunit tagged with a hexahistidine repeat (βHis6, bottom). (B–D) Immunofluorescence images of the dog β1 subunit expressed in CHO cells. CHO WT cells (B), CHO cells expressing SDβ (C), and CHO cells expressing βHis6 in their plasma membranes (D) are depicted. Nuclei were stained with propidium iodide (red). Bars, 40, 30, and 20 μm, respectively. (E) Pull-down assay analysis. Western blots using an antibody against the dog β1 subunit and a His probe reveal, in both cases, the recombinant βHis6 protein (lane 1 and 2), whereas only the dog β1 antibody recognizes the soluble extracellular domain of dog β1 (SDβ, lanes 3 and 4). A mixture of purified SDβ with βHis6 produces the mixed pattern shown in lane 5. The proteins eluted from a pull-down (PD) assay by using βHis6 as bait and soluble SDβ as prey (lane 6) show the same pattern of bands. Only the band corresponding to the bait protein (βHis6) is observed when the pull-down is performed with CHO WT cell supernatant (lane 7). This is confirmed when both pull-downs are blotted to detect the His6 repeat (lanes 8 and 9).