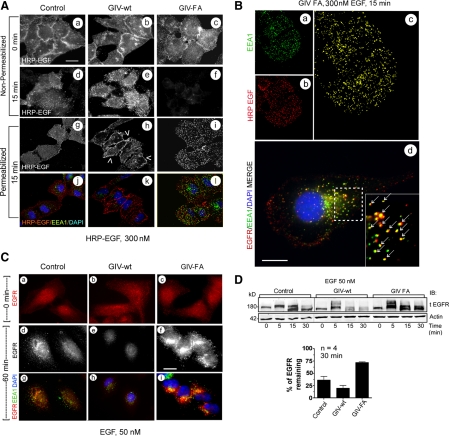
Figure 3.
GIV's GEF function prolongs EGFR localization at the PM but enhances degradation upon internalization. (A) At 15 min after ligand (HRP-EGF) stimulation, ligand-bound EGFR localizes at the PM (e, arrowheads in h) in GIV-wt cells and within intracellular compartments (i and l) in GIV-FA cells. Starved control, GIV-wt, and GIV-FA cells were labeled and stimulated with 300 nM HRP-EGF (equivalent to 50 nM EGF) at 4°C (0 min), washed with PBS, and warmed to 37°C for 15 min. They were then fixed and costained with or without prior permeabilization for HRP, EEA1, and nucleus/DAPI, and visualized by confocal microscopy. Staining for HRP without permeabilization allows selective visualization of ligand-bound receptor at the PM (a–f), whereas permeabilization allows visualization of both the PM and intracellular pools of receptor (g–l). Bar, 10 μm. (B) In GIV-FA cells, EGFR maximally colocalizes with EEA1-positive endosomes at 15 min. GIV-FA cells were stimulated with HRP-EGF for 15 min as in Figure 3A, visualized by confocal microscopy, and analyzed for colocalization of EEA1 (green; a) and HRP (red; b) using Volocity software. The yellow pixels (c) showed significant overlap (Pearson's correlation = 0.45) between HRP-EGF and EEA1. Identical results were obtained when the C terminus of EGFR was stained instead of the ligand (arrows in d). Bar, 10 μm. (C) At 60 min after ligand stimulation, EGFR is virtually undetectable in GIV-wt cells (e and h) but significant staining is seen in GIV-FA cells (f and i) compared with controls (d and g). Cells were stimulated with 50 nM EGF for 60 min and costained for EEA1 (green), tEGFR (red; anti-EGFR cytoplasmic tail), and the nucleus/DAPI (blue). Bar, 10 μm. (D) EGFR degradation is delayed in GIV-FA cells. Serum-starved control, GIV-wt, and GIV-FA HeLa cells were stimulated for 30 min with 50 nM EGF as in Figure 1C and analyzed for total EGFR (tEGFR, anti-EGFR cytoplasmic tail) and actin by immunoblotting (IB; top). Band-shifts and doublets are consistently detected that correlate with phosphorylation of EGFR. Bottom, the amount of receptor (180 kDa, full length) present at 30 min was quantified by Odyssey infrared imaging, normalized to actin, and expressed as percent remaining compared with 0 min. Results are shown as mean ± SEM.