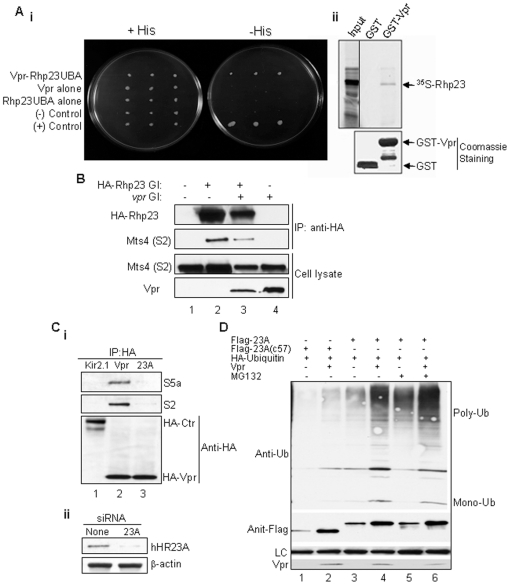
Figure 2. hHR23A is critical for Vpr-proteasome interaction.
A. In vitro and in vivo interactions of Rhp23 with HIV-1 Vpr. (i) Rhp23, a fission yeast homologue of mammalian hHR23A[15], interacts with Vpr in the yeast two-hybrid system. The vpr gene was inserted into the pGBT9 plasmid and rph23 was fused to the activation domain in the pGAD-GH plasmid. The interaction was measured by β-galactosidase assay on cell extracts. (ii) In vitro interaction of Vpr with Rhp23. Bacterial cell lysates over-expressing GST and GST-Vpr proteins were immobilized on GST-agarose beads. In vitro translated 35S-labeled Rhp23 (shown by arrow) was incubated with the immobilized GST and GST-Vpr. The Coomassie staining (low panel) shows total proteins, and autoradiography (top panel) shows bound 35S-labeled Rhp23. B. Interaction of Rph23 with proteasome in the presence or absence of Vpr. HA-Rhp23-carrying plasmid was transfected into fission yeast in the presence or absence of Vpr. Following immunoprecipitation with anti-HA antibody, precipitates were tested with anti-Mts4, which recognizes the 19S regulatory subunit of the proteasome. C.i. Depletion of hHR23A abolished the interaction of Vpr with proteasome in HeLa cells. The HA-Vpr or HA-Kir2.1 (control) expressing vectors were transfected into HeLa cells with (lane marked 23A) or without hHR23A depletion by siRNA. The HA-tagged proteins were pulled down by anti-HA antibody, and then blotted with anti-S2 and anti-S5a antibodies, which recognize S2 and S5a, respectively, of the 19S regulatory subunits of the proteasome. ii. 50 µg of supernatants from lanes 2 (None) and 3 (23A) of a were blotted with anti-Rad23A or β-actin antibody. D. Vpr promotes protein poly-ubiquitination via hHR23A. Flag-tagged hHR23A was co-expressed with HA-ubiquitin in the presence or absence of Vpr in HeLa cells. Forty-eight hours after transfection, cells were collected and cell extracts were subject to Western blot analysis using indicated antibodies. hHR23(c57) is a non-functional mutant derivative of hHR23A [43]. MG132 was used to inhibit the proteasome activities.