Abstract
Mycobacterium tuberculosis causes a variety of host clinical outcomes. We previously showed that M. tuberculosis disrupted in an operon called mce1 proliferates unchecked in BALB/c mouse lungs. The observed outcome could be attributed either to the mutant bacterial burden or to the host immunopathologic response. To differentiate these possibilities, we studied the outcomes of infection in a mouse strain less susceptible to M. tuberculosis than BALB/c--C57BL/6. We found that the mutant infection reached a plateau in the lungs at a rate similar to that of the wild type. All mice infected with the mutant, but only half of the groups of mice infected with the wild type or complemented strain, died by 40 weeks (p<0.05). At 12–21 weeks of infection, histological examination of the lungs of mice infected with the mutant showed a diffuse pattern of lymphocyte infiltration, while that of mice infected with the other strains exhibited a nodular cellular infiltration pattern. Surprisingly, the number of bacilli recovered from the lungs was similar in all three groups. These observations suggest that rather than the bacterial burden, products of the mce1 operon may directly or indirectly modulate the host immune response that is protective to both the tubercle bacilli and the host.
Keywords: Mycobacterium tuberculosis, mce1 operon, latent tuberculosis infection, C57BL/6 mouse
1. Introduction
Mycobacterium tuberculosis remains a major cause of infectious disease-related mortality worldwide. The high worldwide prevalence of M. tuberculosis infection may be attributed to the ability of this organism to establish latent infection. From a reservoir of those with latent tuberculosis infection (LTBI), 2–23% develop clinically overt disease in their lifetime [1]. Factors that predispose a subset of the infected population to develop active disease include both host and bacteria-related characteristics. New strain-related factors that contribute to clinical outcomes in infected but otherwise immunocompetent hosts are beginning to be elucidated.
Shimono et al. reported that a strain of M. tuberculosis disrupted in a 13-gene operon called mce1 was unable to elicit a Th-1 type immune response in immunocompetent BALB/c mice, and failed to enter a stable, persistent state of infection in the mouse lungs [2]. Instead, the mutant continued to proliferate in the lungs until it killed the mouse. The lungs of BALB/c mice infected with the mce1 operon mutant showed aberrant proinflammatory cell migration and failed to generate tightly organized granulomas [2]. The mutant compared to the wild type elicited significantly lower levels of TNFα, IL-6, and MCP-1 by peritoneal macrophages and RAW cells infected ex vivo [2]. These observations led us to speculate that the products of the operon may be important, either directly or indirectly, for inducing proper granuloma formation, which may be protective both to the tubercle bacilli as well as to the mouse.
The mce1 operon is a member of a family of related operons comprised of mce1, 2, 3 and 4 [3]. Each operon consists of genes encoding two putative integral membrane proteins (YrbEA and YrbEB) at the 5'-end, followed by six genes (mceA to F) encoding proteins with hydrophobic stretches at the N-terminus, possibly representing signal sequences [4]. These features are consistent with the location of these proteins in the cell wall, described by Tekaia et al [5] and Chitale et al [6]. Furthermore, the mce1A gene product induces cytoskeletal rearrangement in HeLa cells, as reported by Casali et al. [7]. Joshi et al recently suggested that the Mce1 loci may comprise an ABC transporter, based on the observation that a putative ATPase encoded by RvO655 may interact with a component of Mce1 transmembrane proteins [8]. We have also shown by detailed phyogenomic analyses that the mce1 operon as well as other members of the mce operon family have features of ABC transporters [9].
The observations by Shimono et al were made with BALB/c mice infected with M. tuberculosis via the tail veil [2]. In this mouse model, the effect of the absence of the mce1 operon products on the host response could not be unequivocally distinguished from the effect resulting from increased bacterial burden; all mice died before the mutant bacterial burden stabilized in the mouse lungs. The mce1 operon is negatively regulated by mce1R when M. tuberculosis is intracellular [10]. We recently showed that M. tuberculosis disrupted in mce1R also becomes hypervirulent in BALB/c mice, but for an exact opposite reason [11]. Constitutive expression of the mce1 genes was associated with rapid proliferation of the mutant in the first 4 weeks of infection and the mutant caused massive granuloma formation in mouse lungs [11].
In this study, we wished to determine if the phenotype (enhanced mouse mortality, aberrant proinflammatory cell migration, poor granuloma formation) of the mce1 mutant we observed in BALB/c mice was dependent on the genetic background of the host, the bacterial burden, or a direct or indirect effect of the mce1 operon gene products [2].
2. Materials and methods
2.1. Mice
Six to 8 week-old female C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were housed in a biosafety level 3-bioharzard facility and were kept with sterile water ad libitum, bedding and mouse chow until use. The University of California, Berkeley, Animal Care and Use Committee approved all animal procedures described below.
2.2. Bacterial strains
The mutant strain, disrupted in an mce1 operon gene called yrbE1b (Rv0168), and yrbE1b-complemented strains were derived from wild type H37Rv as described previously by Shimono et al [2]. The disruption of yrbE1b caused downstream mce1 operon genes to be not expressed, and hence this mutant is referred to as an mce1 operon mutant. Before being used to infect mice, the wild type, mce1 mutant, and complemented M. tuberculosis H37Rv strains were passed once in mice and grown at 37°C in liquid Middlebrook 7H9 medium supplemented with 0.02% glycerol, 0.05% Tween 80 and 10% acid-albumin-dextrose complex enrichment (Difco, Becton & Dickinson, Sparks, MD, USA) to mid-log phase.
2.3. Aerosol infection and CFU determination
Mice were placed in the exposure chamber of an airborne infection apparatus (Glas-col Inc., Terre Haute, IN, USA) and exposed to the indicated doses of bacilli. Each M. tuberculosis strain was used to infect a group of 25 mice. The numbers of viable bacteria (colony-forming units or cfu) in the lung, spleen and liver were determined at the indicated time points from serially diluted homogenized organs in 0.9% NaCl-0.05% Tween 80 plated on nutrient Middlebrook 7H11 agar (Difco, Becton & Dickinson, Sparks, MD, USA). The plates were read after 21 days of incubation at 37° C in humidified air. Approximately 250–600 viable bacilli were delivered to the lungs of each mouse as determined by cfu counts 24h post-infection. The data are expressed as the log10 value of the mean number of bacteria recovered per organ (n=3 animals).
2.4. Histology
At the time-points indicated, the left lung from each of the three mice per experimental group was fixed in 10% formaldehyde in phosphate-buffered saline (PBS) pH 7.2. Tissues were embedded in paraffin and sections were made from the superior, middle and inferior area of each lobe, always maintaining the same orientation in order to examine the same pulmonary sections in each mouse. Five-μm thick sections were obtained and stained with hematoxylin-eosin and Ziehl-Neelsen stains. Sections were evaluated by light microscopy by a veterinary pathologist who had no prior knowledge of the experimental groups. Gross examination was performed and images were photographed by a digital camera.
2.5. Statistics
Differences between the mean of M. tuberculosis cfu recovered from each experimental group were compared by Student's t test. Mouse survival was analyzed by the Kaplan-Meier plot. Differences were considered significant at p < 0.05.
3. Results
3.1. Mice infected with the mce1 operon mutant succumbed to infection earlier than mice infected with the wild type strain
Mice were infected via aerosol with 250–600 M. tuberculosis strain H37Rv, mce1 operon mutant, and mce1-complemented bacilli. By 40 weeks, all 10 (or 100%) of the mice in the group infected with the mutant strain died, while half of the mice in groups infected with wild type or complemented strain remained alive. Also, the mutant-infected group started to die earlier (before 10 weeks) than the other two groups (Fig.1). Statistical difference in the mortality rate between mce1 operon mutant group and wild type and complemented mutant groups were p=0.004 and p=0.02, respectively. There was no statistically significant difference in mortality between wild type and complemented mutant groups (p=0.74).
Fig. 1.

Survival kinetics of C57BL/6 mice infected with M. tuberculosis H37Rv. Ten mice in each group were infected via aerosol and followed for the indicated number of weeks. Mice were monitored daily for signs of disease, and moribund mice were killed by cervical dislocation. By 40 wk, all mice infected with the mce1 mutant strain were dead, while a half of wild-type and complemented mutant groups were still alive. Survival curves are significantly different between mce1 mutant and wild-type and mce1 mutant and complemented mutant groups compared by the Kaplan-Meier survival analysis (p< 0.05).
3.2. Determination of cfu in the lung
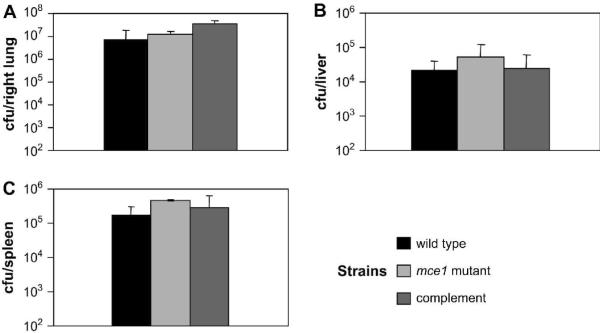
In all three groups of mice, the bacterial strains showed a similar growth pattern in the lungs. The bacillary load in the lungs reached plateau after 3 weeks in all three groups of mice. This plateau phase remained similar for all three strains to about 21 weeks. There was no significant difference in the cfu recovered from lungs among the mouse groups at any time point, except at 24 hours (Fig. 2A). The number of cfu from mce1 operon mutant and complemented mutant groups was higher than the cfu from wild type group at this early time point (Fig. 2A, p<0.05), possibly reflecting differences in the aerosol inoculum. Interestingly, even among moribund animals, there was no significant difference in cfu recovery among the three groups (Fig. 3A). Thus, bacillary load did not correlate with mouse mortality.
Fig. 2.
Time course of infection by aerosol inoculation with M. tuberculosis H37Rv wild type, mce1 mutant, and complemented strains. Recovery of bacteria is determined by cfu enumeration per organ at indicated weeks; the number of cfu is expressed as the mean ± standard deviation (n=3). Wild-type is shown as black squares, mce1 mutant as diamonds, and complemented mutant as white squares.
Fig. 3.
Mycobacterial burden from moribund animals infected via aerosol with M. tuberculosis H37Rv wild type, mce1 mutant and complemented strains. Recovery of bacteria is enumerated by cfu counts per organ from moribund mice near time of death. Number of cfu is expressed as the mean ± standard deviation (n=3). There was no statistically significant difference among the strains.
3.3. Determination of cfu in the liver
All three strains showed a similar growth pattern in the liver, reaching a peak at 6 weeks. After this time point the number of recovered cfu's decreased continuously. We also did not observe significant differences in the number of cfu recovered from the liver at the time of death of the animals (Fig. 3B).
3.4. Determination of cfu in the spleen
The number of cfu recovered from the spleen of the group of mice infected with the mce1 mutant was higher compared with that of mice infected with the complemented-strain at 3 weeks and mice infected with the wild type strain at 6 weeks (Fig.2C, p<0.05). However, after these time points, no significant differences in the cfu recovery were observed (Fig. 2C). Again, there was no significant difference in cfu recovery observed at the time of death of the animals (Fig. 3C).
3.5. Gross organ examination
At all time points (3, 12, and 21 weeks and time of the death of the animals), gross lung examination revealed similar signs of tuberculosis lesions. Granulomas were visible in the lungs by 12 weeks in all mouse groups (data not shown).
3.6. Microscopic examination for acid fast bacilli in the lungs
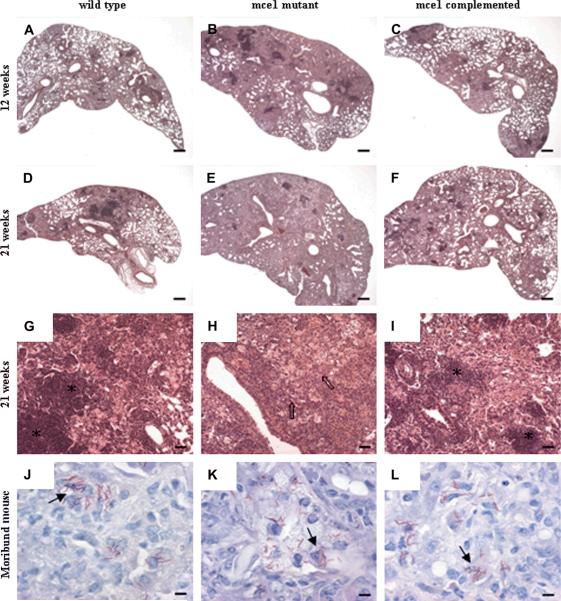
Rare to low number of acid-fast bacilli, usually one per cell, was seen in lung sections at all time points. The number and distribution were similar in the three groups, including in animals at the time of their death. In some sections, the bacilli formed clusters inside macrophages (Fig. 4J, K, L).
Fig. 4.
Representative photomicrographs of lung sections of C57BL/6 mice infected with M. tuberculosis H37Rv wild-type, mce1 mutant and complemented strains. At 12 weeks, more extensive lesions were present in the mce1 mutant group compared to wild-type and complemented groups (hematoxylin/eosin stain; magnification ×20, bar = 300 μm) (A, B, C). At 21 weeks, >90% of the lung parenchyma were affected in mice infected with the mce1 mutant, compared to 50–60% for mice infected with the wild type or complemented mutant (hematoxylin/eosin stain; magnification ×20, bar = 300 μm) (D, E, F). At different magnification (×100, bar = 60 μm) (G, H, I), lesions showed nodular pattern with lymphocyte cuffing around airways or lymphocytes densely packed in clusters in the wild type and complemented groups (asterisks), while in the mce1 mutant group lymphocytes are more dispersed in the parenchyma intermingled with the foamy macrophage population (arrows). Just before death, no difference in the number of bacilli was observed, and they were evenly distributed inside macrophages, in all groups (solid arrows) (Ziehl-Neelsen method; magnification ×1000, bar = 6 μm) (J, K, L).
3.7. Histological examination of the lungs
The histological examination of lung lesions revealed nodular inflammatory cell infiltrates with some areas of interstitial extension at 3 weeks in all three groups. No obvious differences between the groups were observed at this time point (data not shown). By 12 weeks, however, the distribution of the lung lesions in mice infected with the mce1 operon mutant had lost a nodular pattern and became predominantly diffuse, whereas the lesions in mice infected with wild type or complemented strain generally retained the nodular pattern (Fig.4A, B, C). At 12 weeks of infection, more than 80% of the lung parenchyma in the mce1 mutant infected mice was infiltrated with inflammatory cells, obliterating the alveolar airspaces, whereas less than 50% of the lung parenchyma of mice infected with the wild type or complemented strain was affected (Fig. 4A–C). In all three groups, foamy macrophages were predominant, interspersed with clusters of lymphocytes. Any necrosis evident was focal and mild, characterized by clusters of degenerate foamy macrophages associated with a few degenerate neutrophils and sometimes cholesterol clefts of extracellular lipid.
At 21 weeks the lesions in the mce1 mutant-infected group were more diffuse and extensive than those in mice infected with wild type or complemented strains. More than 90% of the lung parenchyma of the mutant infected mice showed pneumonitis, while 50–60% of the lung parenchyma of mice infected with the wild type or complemented strain was affected (Fig. 4D–F). The features associated with more advanced lesion in the mce1 mutant-infected group were greater dispersal of lymphocytes from the tight clusters and more necrosis (Fig.4D to I). Lymphocytes were still evident in clusters around blood vessels, while foamy macrophages remained the predominant inflammatory cells, independent of groups. There were generally more neutrophils infiltrating the lesion and more necrosis in all groups compared to the lesions observed at 12 weeks of infection.
The lesions at the time of death were similar in all groups. The lesions became diffuse and involved nearly the entire lung parenchyma, involving 90% or more of the lung. The extent and severity of the necrosis progressed to moderate or severe. Lymphocytes were further dispersed from clusters with loose perivascular cuffing and were intermingled with the macrophage population; moderate numbers of neutrophils were seen.
4. Discussion
The time of progression to active disease following infection with M. tuberculosis varies greatly in humans, and is likely to be related to factors associated with both the pathogen itself and the host. Previously, we reported the clinical outcomes observed in BALB/c mice following tail vein infection with M. tuberculosis H37Rv disrupted in the mce1 operon [2]. The mce1 operon mutant progressively proliferated in the lungs of BALB/c mice without ever entering a plateau phase and killed the animals more rapidly than did the wild type H37Rv strain. The clinical outcome we observed in BALB/c mice could be attributed to the mce1 mutant strain itself, but it could also have been influenced by the susceptibility of the particular mouse strain that was used, increased bacterial burden, or on the route of delivery of the bacilli. In this present study, we addressed the possible role of the mouse genetic background, bacterial burden, and possible effect of the mce1 operon gene products in the outcome of infection. We used a relatively more resistant mouse strain (C57BL/6). Criteria for clinical outcomes that we compared included 1) bacterial growth rate and burden in the lungs, the liver and the spleen, 2) time to moribund status associated with death, and 3) differences in lung lesions observed histologically.
Routes of infection could also potentially contribute to differences in outcome in mice. Scott & Flynn compared low-dose aerosol infection with high and low-dose IV infection, and found that the low dose allows bacilli to enter a plateau phase in the lungs, independent of the route of infection [12]. Other experiments comparing low-dose aerosol versus IV infection showed bacterial growth to be controlled in the lungs when the aerosol route was used [13]. Gioffré et al showed that at 10 weeks, all mice infected intratracheally with mce1, mce2, and mce3 mutants remained alive, while those infected with the wild type died, suggesting that the mce1 mutant was attenuated in this model [14]. However, the mce1 mutant appeared to be more virulent in lungs if the intraperitoneal route of infection was used [14].
The observation by Gioffré et al that their mce1 mutant was attenuated in BABL/c mouse is not inconsistent with those reported by Shimono et al, who showed that the mce1 mutant was hypervirulent [2]. In the latter study, the mce1 mutant, administered IV to BALB/c mice, did not cause any death until after 25 weeks of infection. Since the animals were followed up to 20 weeks in the study by Gioffré et al, the long term survival outcomes of the animals in the two studies could not be compared [14].
Two other studies reported that the mce1 mutant was attenuated in C57B/6 mice infected IV via tail vein [8, 15]. In both, the investigators used a TRaSH mce1 mutant. The definition of “attenuation” used by Sassetti and Rubin was based on lower cfu counts of the mutant compared to the wild type cultured from the mouse spleen [15] after 8 weeks of infection. Joshi et al also compared cfu counts after 1, 2, 4, and 8 weeks [8]. Our analysis of virulence was based on time to moribund state associated with death and lung lesions (size and cellularity), and we followed the infections for > 40 weeks. As our present study shows, cfu counts are not a reliable indicator of virulence potential of an organism, especially in C56B/6 mouse.
Our study shows that even in C57BL/6 mouse, and with low-dose aerosol delivery of the bacilli, the mce1 operon mutant caused death significantly earlier than did the wild type or complemented strain of H37Rv M. tuberculosis. Interestingly and distinct from the observation made in BALB/c mice, there was no significant difference in the cfu number recovered from any of the three infection groups at later infection time points (>12 weeks) or at the time of death. The mice appeared to be able to control the infection in all 3 groups; the cfu recovery from the lungs reached a plateau after 3 weeks of infection. Thus, death of the mice appears to be related not to the bacterial burden, but more to the lesions associated with the mce1 mutant. This distinction could not be made previously in BALB/c mice [2].
This discordance between bacterial burden and clinical outcome has been observed with other mouse infection models. Mustafa et al showed that mice infected intraperitoneally with a moderate dose of M. tuberculosis strain H37Rv developed slow progressive disease without an increase in bacterial burden [16, 17]. The first phase was characterized by a lack of clinical signs of disease, small granulomas in the lungs and progressive increase in bacterial counts. During the second phase, mice developed signs of disease. The bacterial counts and granuloma size, however, remained stable. The third phase was characterized by severe disease, and a sudden loss of the focal nature of the lesions and spread of inflammatory infiltrates to about 80% of lung parenchyma. This deterioration took place without significant change in the bacterial counts, suggesting that bacterial products, rather than the number of bacteria, contributed to clinical progression to severe disease after a certain point of infection.
Our observations too suggest that the products of the mce1 operon contribute to differences in clinical outcome in infected mice. The products may directly or indirectly induce nodular lung lesion formation (granulomas) that restricts the spread of the tubercle bacilli. Without these products, the mouse exhibits diffuse lung granuloma formation and aberrant proinflammatory cell migration resulting in a faster progression to death [2]. This suggestion is supported by our recent observation that showed that when the mce1 genes are constitutively expressed when the genes are not under negative control by mce1R, an infected mouse develops massive lung granulomas with mixed Th1 and Th2 types of response [11]. Thus, this granulomatous response is opposite in the spectrum of response induced in BALB/c mice by the mce1 operon mutant. As M. tuberculosis requires mammalian hosts for its own long-term survival, it may have evolved to establish a mechanism to temper its virulence to establish persistent infection by inducing an immune response that is advantageous to itself and to the host. The mce1 operon of M. tuberculosis may homeostatically regulate its expression during in vivo infection that allows balanced and stable granulomatous response beneficial to the tubercle bacillus.
5. Acknowledgement
This work was supported by a grant from Ellison Medical Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parrish NM, Dick JD, Bishai WR. Mechanisms of latency in Mycobacterium tuberculosis. Trends Microbiol. 1998;6:107–12. doi: 10.1016/s0966-842x(98)01216-5. [DOI] [PubMed] [Google Scholar]
- 2.Shimono N, Morici L, Casali N, Cantrell S, Sidders B, Ehrt S, Riley LW. Hypervirulent mutant of Mycobacterium tuberculosis resulting from disruption of the mce1 operon. Proc. Natl. Acad. Sci. USA. 2003;100:15918–15923. doi: 10.1073/pnas.2433882100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cole ST, Brosch R, Parkhill J, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 4.Harboe M, Christensen A, Ahmad S, et al. Cross-reaction between mammalian cell entry (Mce) proteins of Mycobacterium tuberculosis. Scand J Immunol. 2002;56:580–7. doi: 10.1046/j.1365-3083.2002.01172.x. [DOI] [PubMed] [Google Scholar]
- 5.Tekaia F, Gordon SV, Garnier T, Brosch R, Barrell BG, Cole ST. Analysis of the proteome of Mycobacterium tuberculosis in silico. Tuber. Lung Dis. 1999;79:329–342. doi: 10.1054/tuld.1999.0220. [DOI] [PubMed] [Google Scholar]
- 6.Chitale S, Ehrt S, Kawamura I, Fujimura T, Shimono N, Anand N, Lu S, Cohen-Gould L, Riley LW. Recombinant Mycobacterium tuberculosis protein associated with mammalian cell entry. Cellular Microbiology. 2001;4:247–254. doi: 10.1046/j.1462-5822.2001.00110.x. [DOI] [PubMed] [Google Scholar]
- 7.Casali N, Konieczny M, Schmidt MA, Riley LW. Invasion activity of a Mycobacterium tuberculosis peptide presented by the Escherichia coli AIDA autotransporter. Infect. Immun. 2002;70:6846–6852. doi: 10.1128/IAI.70.12.6846-6852.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joshi SM, Pandey AK, Capite N, Fortune SM, Rubin EJ, Sassetti CM. Characterization of mycobacterial virulence genes through genetic interaction mapping. Proc Natl Acad Sci U S A. 2006;103:11760–5. doi: 10.1073/pnas.0603179103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casali N, Riley LW. A phylogenomic analysis of the Actinomycetales mce operons. BMC Genomics. 2007;8:60. doi: 10.1186/1471-2164-8-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casali N, White AM, Riley LW. Regulation of the Mycobacterium tuberculosis mce1 operon. J Bacteriology. 2006;188:441–449. doi: 10.1128/JB.188.2.441-449.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uchida Y, Casali N, White A, Morici L, Kendall LV, Riley LW. Accelerated immunopathological response of mice infected with Mycobacterium tuberculosis disrupted in the mce1 operon negative transcriptional regulator. Cell Microbiol. 2007;9:1275–83. doi: 10.1111/j.1462-5822.2006.00870.x. [DOI] [PubMed] [Google Scholar]
- 12.Scott HM, Flynn JL. Mycobacterium tuberculosis in chemokine receptor 2-deficient mice: influence of dose on disease progression. Infect Immun. 2002;70:5946–54. doi: 10.1128/IAI.70.11.5946-5954.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nuermberger EL, Yoshimatsu T, Tyagi S, Bishai WR, Grosset JH. Paucibacillary tuberculosis in mice after prior aerosol immunization with Mycobacterium bovis BCG. Infect Immun. 2004;72:1065–71. doi: 10.1128/IAI.72.2.1065-1071.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gioffre A, Infante E, Aguilar D, et al. Mutation in mce operons attenuates Mycobacterium tuberculosis virulence. Microbes Infect. 2005;7:325–34. doi: 10.1016/j.micinf.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Sassetti CM, Rubin EJ. Genetic requirements for mycobacterial survival during infection. Proc Natl Acad Sci U S A. 2003;100:12989–94. doi: 10.1073/pnas.2134250100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mustafa T, Phyu S, Nilsen R, Jonsson R, Bjune G. A mouse model for slowly progressive primary tuberculosis. Scand J Immunol. 1999;50:127–36. doi: 10.1046/j.1365-3083.1999.00596.x. [DOI] [PubMed] [Google Scholar]
- 17.Mustafa T, Phyu S, Nilsen R, Jonsson R, Bjune G. In situ expression of cytokines and cellular phenotypes in the lungs of mice with slowly progressive primary tuberculosis. Scand J Immunol. 2000;51:548–56. doi: 10.1046/j.1365-3083.2000.00721.x. [DOI] [PubMed] [Google Scholar]