Figure 2.
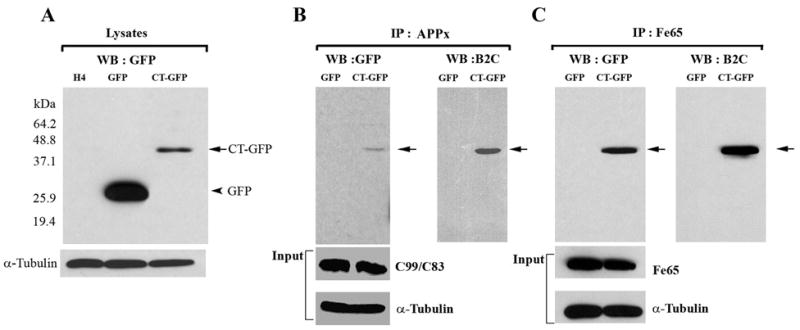
Hirano bodies bind to C-terminal fragments of APP (APP695, APPC99/APPC83 and APPC57-59) and Fe65. A. Cell lysates from wild type, GFP, and CT-GFP stable H4 cells were analyzed by western blot using antibody against GFP. To verify equal loading of the different cell lysates, α-tubulin was employed as a control. The 46 kDa CT-GFP fusion protein (arrow) and 27 kDa GFP protein (arrowhead) are detected as single bands. Marker proteins are 64.2, 48.8, 37.1, 25.9, and 19.4 kDa. B, C. Cell lysates obtained from GFP and CT-GFP stable H4 cells were immunoprecipitated with an antibody against the C-terminal portion of APP (anti-APPx) or with anti-Fe65 antibody, respectively. Products were detected with either anti-GFP or B2C, a monoclonal antibody against 34 kDa protein. To verify equal loading of the different cell lysates, α-tubulin was employed as a control as well as APPx (panel B) and Fe65 (panel C). The immunoprecipitate fraction in panel B contained full length APP (not shown) as well as C99/C83. The results show a specific interaction of APPx (B) and Fe65 (C) with CT-GFP in model Hirano bodies, but not with GFP.
