Figure 6.
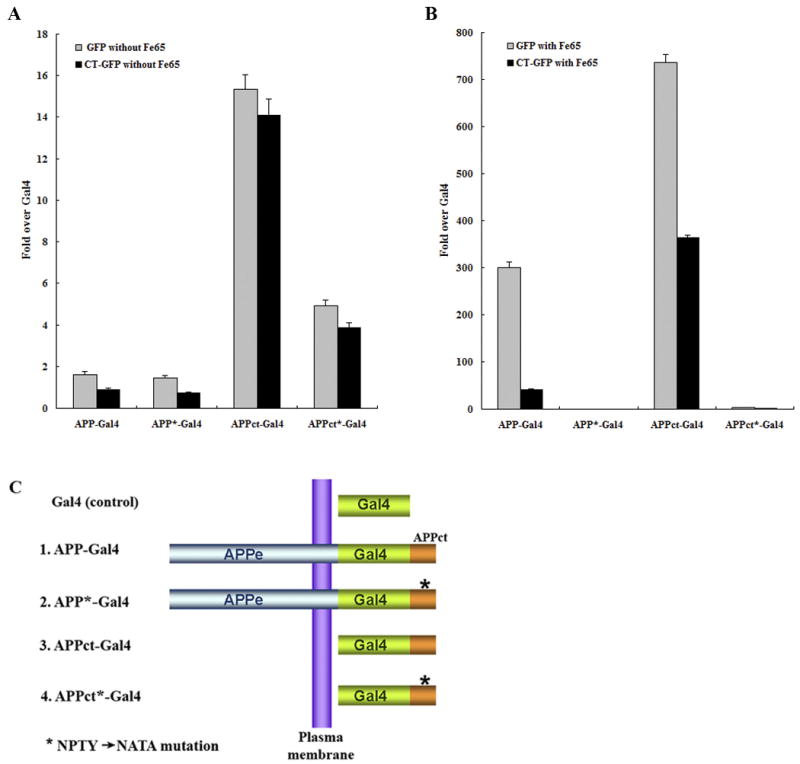
Fe65-dependent transcriptional activity of APP-Gal4 and APPct-Gal4 is down regulated in HEK293T stable cells with Hirano bodies. HEK293T stable cells (1×104 cells) expressing GFP or CT-GFP were co-transfected with 0.1 μg of pG5E1B-luc, 0.02 μg of pRL-TK and one of the following 5 different Gal4 constructs (C): 0.1 μg of pMst (Gal4), 0.1 μg of pMst-APP695 (APP-Gal4), 0.1 μg of pMst-APP695* (APP*-Gal4), 0.1 μg of pMst-APPct (APPct-Gal4), and 0.1 μg of pMst-APPct* (APPct*-Gal4) in the absence (A) or presence (B) of 0.1 μg of HA-Fe65. Plasmids with * contain the NATA mutation instead of NPTY in the C-terminal domain of APP that binds to Fe65. Transactivation activity was normalized to the level of transactivation of control Gal4. APP-Gal4 and APPct-Gal4 induced reporter expression was greatly enhanced by the presence of Fe65 (note the different scale bars in panels A and B). In the presence of Fe65, reporter expression was significantly lower in CT-GFP cells as compared to GFP cells (p < 0.001), and was significantly greater for APP-Gal4 and APPct-Gal4 than for the corresponding constructs with the NATA mutation (marked with *) in the Fe65 binding motif. Data shown are mean ± S.E.M. of triplicate measurements and repeated in three independent experiments.
