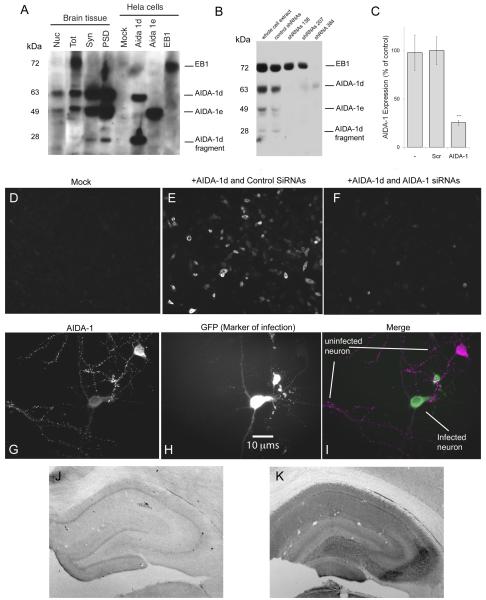
Fig 1.
Characterization of AIDA-1 antibody. The AIDA-1 antibody (Zymed, Invitrogen) was raised against a peptide conjugate corresponding to the sequence RLHDDPPQKPPRSIT at position 172 on AIDA-1. A: Western blot; the AIDA-1 antibody detected for at least 4 different bands corresponding to different AIDA-1 isoforms: 1d (~60 kDa and ~28 kDa), 1e (~49 kDa) and EB-1 (~72 kDa). Nuclei (Nuc) contained prominent bands corresponding to AIDA-1d and AIDA-1e; total lysate (Tot) contained prominent bands corresponding to EB-1, AIDA-1d, and AIDA-1e; and synaptosomes (Syn) and postsynaptic densities (PSD) contained prominent bands corresponding to AIDA-1d, AIDA-1e, and the AIDA-1d fragment. HeLa cells transfected with specific AIDA-1 isoforms confirmed identities of these bands. B: Lentivirus transfection with AIDA-1-specific shRNAs downregulated AIDA expression in cultured hippocampal neurons. ShRNAs targeting bp 138 and 207 on AIDA-1 downregulated 1d and 1e isoforms, while shRNA 384 downregulated all isoforms. C: shRNA 384 reduced expression by 74.5% ± 2.6 (AIDA-1) vs. nonspecific shRNA (SCR) (densitometry, N =8 gels, *** p-value < 0.001). No changes were observed for other markers (data not shown). D-F: HEK-293T cells mock-transfected displayed no staining (D). Cells cotransfected with AIDA-1d and control SiRNAs stained with the Zymed Ab (E), while cells cotranfected with AIDA- and AIDA-1 specific shRNAs showed very little staining (F). G-I: Div 14 hippocampal neurons infected with AIDA-1 specific shRNA 384 showed a marked reduction in AIDA-1 staining (I). AIDA-1 immunoreactivity in the infected (green, H) neuron is reduced in both soma and dendrites vs. an uninfected neuron (magenta, G). J-K: When primary antibody against AIDA-1 is omitted, immunoperoxidase staining in hippocampus is weak, exhibiting a nonspecific pattern (J). A section with primary antibody added displays high levels of staining (K). Contrast in J is greatly enhanced to increase visibility.