Abstract
In the fasting state, approximately 83% of glucose uptake occurs via non-insulin mediated mechanisms. A widely accepted static rate for non-insulin mediated glucose uptake (NIMGU) is 1.62 mg/Kg ·min−1. To investigate the variability of NIMGU, we examined differences by glucose tolerance, sex, age, race (American Indian/African American/Caucasian) and adiposity in 616 volunteers (including individuals with normal and impaired glucose regulation and diabetes) using data from euglycemic hyperinsulinemic clamp experiments. NIMGU was determined by plotting basal glucose output and insulin action against fasting and steady state clamp insulin. The intercept with the Y-axis after extrapolation was interpreted as NIMGU at zero insulin. Body composition was determined by dual-energy X-ray absorptiometry and glucose regulation by a 75 gram oral glucose tolerance test. Energy expenditure was measured by indirect calorimetry in a metabolic chamber. In individuals with normal glucose regulation (NGR, n=385), NIMGU was 1.63 mg/kgEMBS (fat free mass + 17.7 kg) ·min−1 (95% CI 1.59, 1.66). NIMGU increased with impaired glucose regulation and diabetes (IGR: n=189, 1.67 (1.62, 1.72); DM: n=42, 2.39 (2.29, 2.49), p<0.0001 across groups). NIMGU did not differ by sex (p=0.13), age (p=0.22) or race (p=0.06), however NIMGU was associated with % body fat ((PFAT) r2=0.04; p<0.0001). Further, NIMGU was positively associated with 24 h and sleep energy expenditure (r2=0.002, p=0.03; r2=0.01, p<0.01). Extrapolated NIMGU in individuals with NGR is remarkably consistent with previously published data. Our results indicate that NIMGU is associated with adiposity. NIMGU increases with declining glucose tolerance perhaps to preserve glucose uptake during increased insulin resistance.
INTRODUCTION
In the human body, glucose uptake is accomplished via two mechanisms, insulin mediated glucose uptake (IMGU), which occurs only in insulin-sensitive tissues (i.e. liver, muscle and adipocytes) and non-insulin mediated glucose uptake (NIMGU), which occurs in both insulin-sensitive and non-insulin-sensitive tissues (i.e., brain, blood cells, nerve, etc.). As early as 1934, Soskin et al. provided evidence for a mechanism of glucose disposal independent of insulin in pancreatectomized dogs [1]. In experimental models, NIMGU has been defined as uptake of glucose at zero insulin concentrations [2]. In the fasting state NIMGU accounts for about 83% of whole body glucose disposal [3]. Gottesman et al. investigated NIMGU in 16 lean non-diabetic individuals via various insulin infusions after somatostatin induced suppression of endogenous insulin release. In the euglycemic state, NIMGU was 1.62 mg/kg·min−1 which became a widely accepted static rate for NIMGU [4]. How the rate of NIMGU might change in the presence of hyperglycemia is unclear. Forbes at al. reported a significantly lower rate of NIMGU in patients with type 2 diabetes mellitus compared to healthy controls [5]. However, others report no significant differences in NIMGU in diabetic versus non-diabetic patients at similar glucose levels [6,7]. In contrast, Capaldo et al. demonstrated that hyperglycemia increased insulin independent peripheral glucose disposal in individuals with diabetes versus controls [8]. In most of these studies, NIMGU was assessed via suppression of endogenous insulin with somatostatin, however, these studies all involved a small number of study subjects.
We assessed NIMGU rates by extrapolation of clamp data in 616 volunteers and examined differences by glucose tolerance, sex, age, race and adiposity in a population of American Indians, African Americans and Caucasians. Because glucose induced glucose uptake (via mass action) might be expected to induce futile cycles (such as the Cori cycle) which would influence metabolic rate [9], we investigated whether NIMGU was also related to energy expenditure.
METHODS
Volunteers participated in a longitudinal study of predictors of type 2 diabetes. All subjects were free of other medical diseases as determined by laboratory testing, history and physical. Subjects were not taking any medications and were non-smokers. Volunteers were admitted to the clinical research unit and placed on a weight maintaining diet for at least 3 days prior to any metabolic testing. For this substudy analysis, 616 subjects were selected who had complete data for anthropometry as measured by dual energy x-ray absorptiometry (DXA) (DPX-L; Lunar Radiation, Madison, WI), glucose regulation status determined by oral glucose tolerance testing (OGTT) and insulin action (M) evaluated using the hyperinsulinemic euglycemic glucose clamp technique. All subjects provided written informed consent. The protocol and consent were approved by the Institutional Review Board of the National Institute of Diabetes Digestive and Kidney Disease.
Oral glucose tolerance test
After an overnight fast subjects were given a 75 g oral glucose load. Blood samples were drawn at 0 (G0), 30 (G30), 60 (G60), 120 (G120), and 180 (G180) min for measurement of plasma glucose and insulin concentrations. According to results of the OGTT, subjects were categorized as either having normal glucose regulation (NGR, fasting plasma glucose (FPG) < 5.6 mmol/l and 2 hour plasma glucose (2hPG) < 7.8 mmol/l), impaired glucose regulation (IGR, FPG ≥ 5.6 mmol/l and < 7.0 mmol/l and/or 2hPG ≥ 7.8 mmol/l and < 11.1 mmol/l) or type 2 diabetes (DM, FPG ≥ 7.0 mmol/l and/or 2hPG ≥ 11.1 mmol/l) per ADA 2003 criteria [10]. Incremental area under the curve (iAUC) for glucose was calculated by: (((G0+G30)/2)*30)+(((G60+G120)/2)*60)+(((G120+G180)/2)*60)-(G0*G180). Plasma insulin concentrations were measured by three different radioimmunoassays used over time in our lab: modified Herbert-Lau assay [11], Concept 4 (Concept 4; ICN, Costa Mesa, CA) and Access (Beckman Instruments. Insulin assays). All measurements of insulin were normalized to the original radioimmunoassay (modified Herbert-Lau assay) using regression equations. Plasma glucose concentrations were determined by the glucose oxidase method (Beckman Instruments, Fullerton, CA).
Hyperinsulinemic euglycemic glucose clamp
Details of the insulin clamp technique have been previously described [12]. Briefly, after an overnight fast a catheter was placed in the antecubital vein and a primed (1.11 MBq) continuous [0.0111 MBq/min] 3-[3H] glucose infusion was started to determine endogenous glucose production (EGP). Two hours after beginning infusion of the 3-[3H] glucose, a primed continuous insulin infusion was administered at the rate of 40 mU/m2/min for 100 min. After the start of the insulin infusion, plasma glucose concentrations were measured every 5 min and a variable infusion of 20% dextrose was used to maintain glucose at 5.6 mmol/l. Basal glucose output (BGO) was calculated during the fasting state as the 3-[3H] glucose infusion rate divided by the steady-state plasma 3-[3H] glucose specific activity (measured with Beckman LS6500 scintillation counter; Beckman Instruments, Fullerton, CA). During the insulin clamp, endogenous glucose production was calculated from Steele’s non-steady state [13]. The rate of glucose disposal (M) was defined as the average sum of glucose infusion rate (GIR) and EGP during the last 40 min of the insulin infusion and was corrected for both steady-state plasma insulin levels and endogenous glucose production. M values were normalized to estimated metabolic body size (EMBS: fat free mass + 17.7 kg) [14].
Measurement of energy expenditure
Energy expenditure (EE) was measured in the respiratory chamber as previously described [15]. Briefly, after an overnight fast, study volunteers entered the chamber at 0645 h and remained therein for 23 h. Meals were provided at 0800, 1130, 1700 and 2000 h. Energy content of provided meals was only 80% of the weight maintaining diet because of the confinement within the chamber. During constant flow of fresh air through the chamber, CO2 production and O2 consumption were measured and calculated every 15 min and extrapolated for a 24 h interval. Radar sensors were used to detect spontaneous physical activity expressed as percentage of time over the 23-h period in which activity was evaluated. Energy expenditure during sleep (SLEEPEE) was defined as the average energy expenditure of all 15 min periods between 2330 and 0500 h during which spontaneous activity was <1.5%.
Statistical analysis
NIMGU was determined by plotting BGO and M-values (Y-axis) against fasting and steady state clamp insulin (X-axis) for each volunteer. The intercept of this line with the Y-axis after extrapolation was interpreted as NIMGU, i.e. the glucose uptake at zero insulin concentrations (Figure 1). Subject characteristics are depicted as mean ± SD or median (25th to 75th percentile). Normally distributed variables were analyzed by Student’s test and for multiple groups by One-Way ANOVA. Skewed variables were analyzed by the Kruskall-Wallis test. Linear regression models were used to calculate least square means and 95% confidence intervals for NIMGU after adjusting for sex, age, race, % body fat (PFAT) and glucose regulation status. Linear regression models adjusted for fat mass, fat-free mass, age, race and glucose regulation status were also used to test the association of NIMGU with energy expenditure. For comparison between multiple groups, p-values were adjusted using the Tukey correction. Alpha was set at p<0.05.
Figure 1.
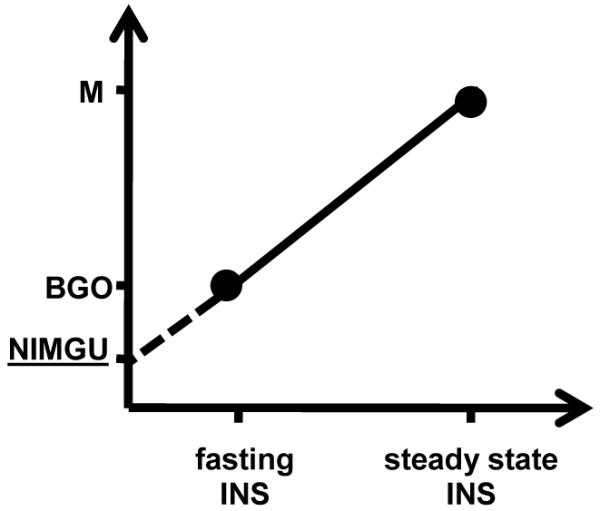
Schematic diagram depicting the determination of NIMGU. By plotting BGO- and M-values on the Y-axis against fasting and steady state clamp insulin on the X-axis we created two data points per individual. NIMGU was then determined via extrapolation of the linear slope to the intercept with the Y-axis at virtually zero plasma insulin concentrations (dashed line).
RESULTS
Subject characteristics
Anthropometric and metabolic characteristics of the 616 study volunteers are depicted in Table 1. BGO was higher in individuals with type 2 diabetes compared to individuals with normal or impaired glucose regulation. BGO was lowest in American Indians and highest in African Americans and was higher in women compared to men. Fasting insulin concentrations were higher in women, American Indians and increased with worsening glucose tolerance. Steady state clamp insulin concentrations were highest in American Indians and lowest in Caucasians. As expected, M was lower in American Indians and individuals with type 2 diabetes. In regression models, BGO was positively and M negatively associated with PFAT (data not shown).
Table 1.
Characteristics of Study Subjects
| Sex |
Race |
Glucose Regulation |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | p | American Indian |
African American |
Caucasian | p | NGR | IGR | DM | p | |
| N | 353 (13) | 263 (29) | - | 470 (40) | 35 (0) | 111 (2) | - | 385 | 189 | 42 | - |
| AGE | 28±7 | 26(22-31) | NS | 27±6 | 26(22-31) | 29±7 | **** | 25(21-31) | 29.6±6.2 | 28.3±6.4 | **** |
| BMI | 33.0±7.7 | 35.6±8.6 | **** | 34.6±7.8 | 32.0±7.7 | 33.0±9.5 | NS | 32.2±7.7 | 37.0±8.4 | 38.5±6.3 | **** |
| PFAT | 27.9±7.9 | 39.2(35.3-43.3) | **** | 33.6±8.4 | 25.6±9.3 | 29.3±10.5 | **** | 30.0±9.1 | 35.7±8.2 | 38.6±6.2 | **** |
| FPG ° | 4.9±0.7 | 5.4±1.2 | ** | 5.2±1.1 | 5.0±0.4 | 5.0±0.5 | NS | 4.8±0.4 | 5.4±0.5 | 7.2±2.3 | **** |
| 2hPG ° | 6.7±2.2 | 8.1±3.0 | **** | 7.6±2.8 | 6.1±1.7 | 6.4±1.7 | ** | 6.0(5.2-6.8) | 8.4±1.4 | 14.4±3.1 | **** |
| FPI ° | 236±132 | 292±146 | ** | 278±139 | 125(104-222) | 171(111-236) | **** | 195(132-271) | 297(195-392) | 410±160 | **** |
| SSPI ° | 958±285 | 1035±333 | * | 1021±313 | 847(757-944) | 833(695-1028) | ** | 952±306 | 1056±313 | 1097±264 | **** |
| BGO ° | 1.9±0.3 | 2.0±0.4 | ** | 1.9±0.3 | 2.1±0.2 | 2.0±0.3 | **** | 1.9±0.3 | 1.9±0.3 | 2.3±0.6 | **** |
| M ° | 2.8(2.2-3.9) | 2.7(2.2-3.2) | NS | 2.5(2.1-3.1) | 4.0±1.4 | 3.8±1.4 | **** | 3.0(2.4-4.0) | 2.2(2.9-2.8) | 2.1±0.4 | **** |
Variables are depicted as mean±SD or medians (25th to 75th percentile).
NGR= normal glucose regulation, IGR= impaired glucose regulation, DM= type 2 diabetes, AGE in years, BMI= Body Mass Index (kg/m2), PFAT= percent body fat, FPG= fasting plasma glucose (mmol/l), 2 h PG= 2 hour plasma glucose (mmol/l), FPI= fasting plasma insulin (pmol/l) , SSPI= clamp steady state plasma insulin (pmol/l), BGO= basal glucose output (mg/kgEMBS·min−1), M= insulin action (mg/kgEMBS·min−1), EMBS= estimated metabolic body size (fat free mass + 17.7), numbers in parenthesis represent diabetic individuals
p-values are adjusted for glucose regulation status in Sex and Race groups due to the skewed distribution of diabetic individuals
NS p>0.05
p<0.05
p≤0.01
p≤0.001
p≤0.0001
NIMGU
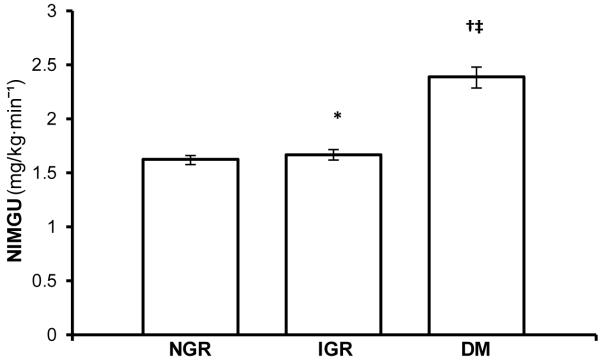
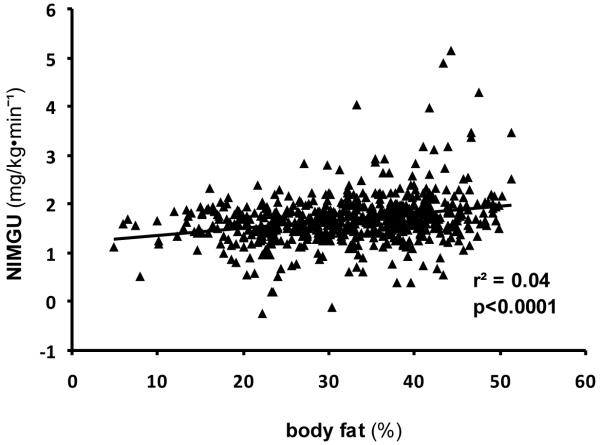
NIMGU was lowest in the NGR group and increased with worsening glucose regulation status (NGR (n=385), 1.63 mg/KgEMBS·min−1 (95 % CI 1.59, 1.66); IGR (n=189), 1.67 mg/KgEMBS·min−1 (95 % CI 1.62, 1.72); DM (n=42), 2.39 mg/KgEMBS·min−1 (95 % CI 2.29, 2.49), p=<0.0001 for trend across groups) (Figure 2). NIMGU did not differ significantly between NGR and IGR groups (p=0.17) but was significantly higher in the DM group compared to IGR and DM (p<0.0001 for each comparison). Within the NGR group, iAUC during the OGTT was a significant positive determinant of NIMGU (p<0.01). In the whole cohort, NIMGU did not differ by sex (p=0.13), age (p=0.22) or race (p=0.06). However, NIMGU was significantly positively associated with PFAT (r2=0.04; p<0.0001; Figure 3). To further explore an association to body fat distribution we included waist, thigh circumference and waist/thigh ratio respectively in the model instead of PFAT. No significant association was found. In the subset of individuals with measurements of EE (n=342: NGR=219, IGR=102, DM=21), NIMGU was positively associated with 24EE and SLEEPEE (additional r2 explained over the reduced model=0.002, p=0.03; r2=0.01, p<0.01). Further, in models adjusted for age and sex, higher NIMGU predicted the development of diabetes (hazard rate ratio (HR) 2.45 (1.10; 5.46), p=0.03). However, including PFAT in the model showed that NIMGU as a predictor of type 2 diabetes is partially dependent on PFAT (HR 1.95 (0.87; 4.34), p=0.10).
Figure 2.
Mean NIMGU in individuals with normal glucose regulation (NGR), impaired glucose regulation (IGR) and type 2 diabetes mellitus (DM). Error bars show 95 % CI. * Not significant vs. NGR; † p<0.0001 vs. NGR; ‡ p<0.0001 vs. IGR
Figure 3.
Association of NIMGU and body fat in the whole study cohort (616 individuals)
DISCUSSION
In this large dataset, including American Indians, African Americans and Caucasians, we found that extrapolated NIMGU in the NGR group was strikingly similar to that measured in previous studies. NIMGU increased with worsening glucose regulation, was associated with body fat, but not age, and did not differ by sex or race.
A limitation of our approach is that the calculation of NIMGU was an extrapolation to zero insulin concentrations based on BGO and M values plotted against respective insulin concentrations. Since each NIMGU (intercept on the Y-axis) is dependent on only two points per individual, greater variation in one variable (in this case the M value) would more greatly affect the value of the intercept. Specifically, a relatively greater decline in M as may occur with increased adiposity or worsening glucose tolerance would result in a higher intercept. In those with DM, the intercept would be expected to increase even more as BGO increases. However, our results for NIMGU in NGR subjects were remarkably consistent with previously published data of NIMGU assessment during somatostatin induced insulin suppression (1.63 mg/kg·min−1 vs. 1.62 mg/kg·min−1) (4). Likewise, Baron et al. reported a similar whole body glucose uptake of 1.83 mg/kg·min−1 at insulinopenia and euglycemia in 6 volunteers [3]. Further, this group investigated NIMGU in 7 diabetic subjects and 7 control subjects. They reported a slight elevation of NIMGU in subjects with type 2 diabetes compared to control subjects at matched plasma glucose concentrations. Although this did not reach significance, it is consistent with our observation of an increase in NIMGU in states of declining insulin sensitivity [6]. This is further supported by the positive association of NIMGU and glucose iAUC from our OGTT measurements. Moreover, NIMGU was significantly associated with both 24EE and SLEEPEE measured on a separate day from the euglycemic hyperinsulinemic clamp. NIMGU would be expected to fuel futile cycles such as the Cori Cycle [9], representing the degradation of glucose to C3-molecules with transfer of the C3-molecules back to the liver for recycling via gluconeogenesis. As an increase in futile cycling would lead to an increase in metabolic rate, the association of NIMGU with EE supports our supposition that NIMGU represents a physiologic measure of glucose uptake. A strength of our study is that this analysis was performed on a large group of individuals which would not be feasible using the method of somatostatin induced suppression of insulin secretion. Although the nature of glucose disposal at low insulin concentrations is not yet fully explored, previous reports including one study by Gottesman et al using somatostatin to suppress insulin secretion provide evidence for a near linear relation of glucose disposal at low plasma insulin levels [4,16]. Hence, given the large number of study subjects we believe that these results further the understanding of the importance of NIMGU.
Others have evaluated insulin independent glucose uptake using glucose effectiveness or Sg derived from the minimal model estimation [17]. When using this term, in contrast to our findings, studies in the past have illustrated that Sg was lower in diabetic individuals compared to healthy controls [18,19]. Moreover, Martin et al. found that lower Sg predicted the development of diabetes [20]. However, Sg and NIMGU are not equivalent as Sg includes the effect of basal insulin on glucose uptake while NIMGU does not. In addition, Sg determination via the minimal model approach seems to be controversial as it is reported to be either under or overestimated in individuals with impaired insulin action depending on the study [21,22]. Specifically, Finegood et al. reported that Sg between groups with significantly different insulin secretory function should be interpreted cautiously as reduced Sg in diabetic subjects is likely due to an artifact of the minimal model [23]. In support of our data, several other studies found that Sg was significantly higher in insulin resistant or diabetic states, interpreting this finding as a compensatory mechanism to impaired insulin-mediated glucose uptake (IMGU) [8,24].
Glucose uptake into cells is accomplished by two transporter systems, namely glucose transporters (GLUT) and sodium-glucose cotransporters. In individuals with impaired glucose tolerance or type 2 diabetes mellitus, trafficking of the insulin sensitive GLUT-4 transporter is decreased, possibly leading to enhanced non-insulin mediated pathways to preserve glucose entry into the cell. This compensatory mechanism could include an increase in intrinsic activity of insulin-independent glucose mediators such as GLUT-1 and/or sodium-glucose cotransporters. Interestingly, Lopez et al. recently investigated the association of IMGU and Sg in offspring of parents with type 2 diabetes (FH+) and offspring with no history of parental diabetes (FH-) [25]. They found that the positive correlation of IMGU and Sg in FH- subjects is not present in FH+ subjects, suggesting independent regulatory mechanisms of glucose uptake pathways in persons at risk for diabetes. Further, they found a positive association between Sg and BMI, which is in accordance with the strong association of NIMGU and PFAT in our data. We also included waist and thigh circumference and waist/thigh ratio respectively in the linear model instead of PFAT to explore a possible association of NIMGU to body fat distribution. We did not find an association of NIMGU with waist and thigh circumference or waist/thigh ratio. However, trials with more precise measurements of body fat distribution could be useful to address the role specifically of metabolically active abdominal fat. With support of the literature, our results in a large group of individuals provide further evidence of elevated NIMGU in insulin resistant states. Therefore, non-insulin mediated glucose uptake mechanisms may play an important compensatory role for plasma glucose clearance in patients with type 2 diabetes.
CONCLUSIONS
We found that by using a method of extrapolation to determine NIMGU, the calculated value in individuals with NGR was identical to that determined in previous studies using somatostatin-induced insulinopenia. NIMGU was increased in individuals with type 2 diabetes and was related to percent body fat. Furthermore, NIMGU was an independent predictor of EE. These results indicate that increasing adiposity and associated insulin resistance result in upregulation of insulin independent alternative pathways of glucose uptake potentially as a compensatory measure to ensure sufficient glucose flux into the cell.
Acknowledgements
We thank all study volunteers for their contribution to this study. We also thank the staff of the Clinical Research Unit on the 5th floor of the Phoenix Indian Medical Center. This study was supported by the intra-mural research program of the NIDDK.
Footnotes
Conflict of Interest: The authors report no conflict of interest or financial interest relevant to this article.
Institutional Approval: The protocol and consent were approved by the Institutional Review Board of the National Institute of Diabetes Digestive and Kidney Disease.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Soskin S, Allweiss MD, Cohn DJ. Influence of the pancreas and the liver upon the dextrose tolerance curve. American Journal of Physiology. 1934;109:155–165. [Google Scholar]
- [2].Wiernsperger NF. Is non-insulin dependent glucose uptake a therapeutic alternative? Part 1: physiology, mechanisms and role of non insulin-dependent glucose uptake in type 2 diabetes. Diabetes Metab. 2005;31:415–426. doi: 10.1016/s1262-3636(07)70212-4. [DOI] [PubMed] [Google Scholar]
- [3].Baron AD, Brechtel G, Wallace P, Edelman SV. Rates and tissue sites of non-insulin- and insulin-mediated glucose uptake in humans. Am J Physiol. 1988;255:E769–E774. doi: 10.1152/ajpendo.1988.255.6.E769. [DOI] [PubMed] [Google Scholar]
- [4].Gottesman I, Mandarino L, Gerich J. Estimation and kinetic analysis of insulin-independent glucose uptake in human subjects. Am J Physiol. 1983;244:E632–E635. doi: 10.1152/ajpendo.1983.244.6.E632. [DOI] [PubMed] [Google Scholar]
- [5].Forbes A, Elliott T, Tildesley H, Finegood D, Meneilly GS. Alterations in non-insulin-mediated glucose uptake in the elderly patient with diabetes. Diabetes. 1998;47:1915–1919. doi: 10.2337/diabetes.47.12.1915. [DOI] [PubMed] [Google Scholar]
- [6].Baron AD, Kolterman OG, Bell J, Mandarino LJ, Olefsky JM. Rates of noninsulin-mediated glucose uptake are elevated in type II diabetic subjects. J Clin Invest. 1985;76:1782–1788. doi: 10.1172/JCI112169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Garcia-Estevez DA, Araujo-Vilar D, Cabezas-Cerrato J. Non-insulin-mediated glucose uptake in several insulin-resistant states in the postabsortive period. Diabetes Res Clin Pract. 1998;39:107–113. doi: 10.1016/s0168-8227(97)00124-1. [DOI] [PubMed] [Google Scholar]
- [8].Capaldo B, Santoro D, Riccardi G, Perrotti N, Sacca L. Direct evidence for a stimulatory effect of hyperglycemia per se on peripheral glucose disposal in type II diabetes. J Clin Invest. 1986;77:1285–1290. doi: 10.1172/JCI112432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zawadzki JK, Wolfe RR, Mott DM, et al. Increased rate of Cori cycle in obese subjects with NIDDM and effect of weight reduction. Diabetes. 1988;37:154–159. doi: 10.2337/diab.37.2.154. [DOI] [PubMed] [Google Scholar]
- [10].Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26(Suppl 1):S5–20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- [11].Herbert V, Lau KS, Gottlieb CW, Bleicher SJ. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965;25:1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- [12].Lillioja S, Mott DM, Howard BV, et al. Impaired glucose tolerance as a disorder of insulin action. Longitudinal and cross-sectional studies in Pima Indians. N Engl J Med. 1988;318:1217–1225. doi: 10.1056/NEJM198805123181901. [DOI] [PubMed] [Google Scholar]
- [13].Steele R. Influences of Glucose Loading and of Injected Insulin on Hepatic Glucose Output. Annals of the New York Academy of Sciences. 1959;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- [14].Lillioja S, Bogardus C. Obesity and insulin resistance: lessons learned from the Pima Indians. Diabetes Metab Rev. 1988;4:517–540. doi: 10.1002/dmr.5610040508. [DOI] [PubMed] [Google Scholar]
- [15].Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest. 1986;78:1568–1578. doi: 10.1172/JCI112749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Koska J, Ortega E, Bunt JC, et al. The effect of salsalate on insulin action and glucose tolerance in obese non-diabetic patients: results of a randomised double-blind placebo-controlled study. Diabetologia. 2009;52:385–393. doi: 10.1007/s00125-008-1239-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bergman RN. Lilly lecture 1989. Toward physiological understanding of glucose tolerance. Minimal-model approach. Diabetes. 1989;38:1512–1527. doi: 10.2337/diab.38.12.1512. [DOI] [PubMed] [Google Scholar]
- [18].Ward GM, Weber KM, Walters IM, et al. A modified minimal model analysis of insulin sensitivity and glucose-mediated glucose disposal in insulin-dependent diabetes. Metabolism. 1991;40:4–9. doi: 10.1016/0026-0495(91)90183-w. [DOI] [PubMed] [Google Scholar]
- [19].Taniguchi A, Nakai Y, Fukushima M, et al. Pathogenic factors responsible for glucose intolerance in patients with NIDDM. Diabetes. 1992;41:1540–1546. doi: 10.2337/diab.41.12.1540. [DOI] [PubMed] [Google Scholar]
- [20].Martin BC, Warram JH, Krolewski AS, et al. Role of glucose and insulin resistance in development of type 2 diabetes mellitus: results of a 25-year follow-up study. Lancet. 1992;340:925–929. doi: 10.1016/0140-6736(92)92814-v. [DOI] [PubMed] [Google Scholar]
- [21].Cobelli C, Bettini F, Caumo A, Quon MJ. Overestimation of minimal model glucose effectiveness in presence of insulin response is due to undermodeling. Am J Physiol. 1998;275:E1031–E1036. doi: 10.1152/ajpendo.1998.275.6.E1031. [DOI] [PubMed] [Google Scholar]
- [22].Caumo A, Vicini P, Cobelli C. Is the minimal model too minimal? Diabetologia. 1996;39:997–1000. doi: 10.1007/BF00403922. [DOI] [PubMed] [Google Scholar]
- [23].Finegood DT, Tzur D. Reduced glucose effectiveness associated with reduced insulin release: an artifact of the minimal-model method. Am J Physiol. 1996;271:E485–E495. doi: 10.1152/ajpendo.1996.271.3.E485. [DOI] [PubMed] [Google Scholar]
- [24].Henriksen JE, Alford F, Handberg A, et al. Increased glucose effectiveness in normoglycemic but insulin-resistant relatives of patients with non-insulin-dependent diabetes mellitus. A novel compensatory mechanism. J Clin Invest. 1994;94:1196–1204. doi: 10.1172/JCI117436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lopez X, Bouche C, Tatro E, Goldfine AB. Family history of diabetes impacts on interactions between minimal model estimates of insulin sensitivity and glucose effectiveness. Diabetes Obes Metab. 2009;11:123–130. doi: 10.1111/j.1463-1326.2008.00913.x. [DOI] [PubMed] [Google Scholar]